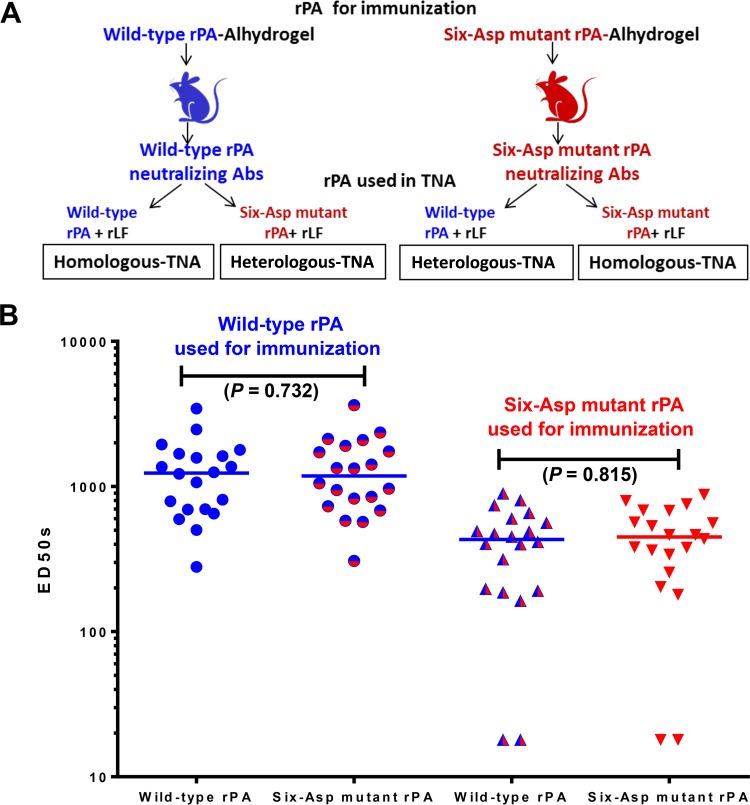
FIG 3.
Ability of sera from mice immunized with either wild-type rPA vaccine or six-Asp mutant rPA vaccine to neutralize the homologous and heterologous toxins. (A) A schematic diagram of the experimental design is shown. (B) Two groups of 20 mice each were immunized with either freshly prepared wild-type or six-Asp mutant rPA Alhydrogel formulations (10 μg of rPA/mouse). At 28 days postimmunization, mice were bled, and serum samples were analyzed in a TNA assay which utilized either the homologous or the heterologous toxin as described in Materials and Methods. The graph shows the TNA titers (expressed as ED50s) for each mouse. The x axis indicates the type of rPA used to prepare the LT used in the TNA assay. The horizontal lines represent the medians for the groups. A nonparametric Mann-Whitney test was performed to calculate the statistical significance using GraphPad Prism software. The P values are indicated. This figure is representative of three independent immunization studies. Abs, antibodies; rLF, recombinant lethal factor.