Abstract Abstract
This population pharmacokinetics (PK) analysis characterized the PK of the oral soluble guanylate cyclase stimulator riociguat in patients with renal or hepatic impairment and determined whether smoking affects riociguat dosing. Two phase 1 studies were performed in patients with renal impairment (n = 72, of whom 11 were smokers), and two were performed in those with hepatic impairment (n = 64, of whom 12 were smokers). Plasma and urine samples were collected after a single oral dose of riociguat 1.0 or 0.5 mg. Nonlinear mixed-effects modeling was used to develop a combined, two-compartment population PK model for riociguat and its main metabolite, M1. Riociguat and M1 clearance was split into renal and nonrenal parts; the nonrenal part for riociguat was divided into metabolism to M1 and a metabolic (nonrenal) part. Total clearance of riociguat was 1.912 L/h. The main route of riociguat clearance is metabolism to M1 (1.2 L/h). In this model, hepatic function biomarkers or Child-Pugh classification had no significant effect on riociguat or M1 clearance. Nonrenal (nonmetabolism) riociguat clearance was similar in all groups. Renal clearance (0.242 L/h) contributed less to riociguat total clearance, mainly determined by glomerular filtration (0.174 L/h). Renal impairment reduced riociguat and M1 clearance. Hepatic or renal impairment had limited effects on total exposure to riociguat. However, individual dose adjustment of riociguat should be administered with particular care in patients with moderate hepatic or renal impairment. Riociguat is not recommended in severe hepatic or renal impairment. Smoking reduced riociguat exposure by significantly increasing metabolism to M1.
Keywords: pulmonary arterial hypertension, chronic thromboembolic pulmonary hypertension, soluble guanylate cyclase stimulator, drug exposure
Pulmonary hypertension is associated with endothelial dysfunction, impaired synthesis of nitric oxide (NO), and insufficient stimulation of the NO–soluble guanylate cyclase (sGC)–cyclic guanosine monophosphate (cGMP) pathway.1-3 Riociguat, the first member of a novel class of therapeutics called sGC stimulators, has a dual mode of action.2-5 In addition to sensitizing sGC to endogenous NO by stabilizing NO-sGC binding, it also directly stimulates sGC via a different binding site, independently of NO.2,3 Results from animal studies have demonstrated that sGC stimulation and increases in cGMP lead to vasodilatory, antifibrotic, antiproliferative, and anti-inflammatory effects.2-4,6-12 Furthermore, riociguat is the first drug to consistently demonstrate robust efficacy in two separate pulmonary hypertension indications: pulmonary arterial hypertension (PAH) and chronic thromboembolic pulmonary hypertension (CTEPH).13,14
Riociguat is licensed in more than 50 countries, including the United States, those of the EU, Canada, and Japan for the treatment of PAH and persistent/recurrent CTEPH after surgical treatment or inoperable CTEPH.15-17 Among patients with PAH or CTEPH suitable for riociguat treatment there are likely to be individuals with renal or hepatic impairment. The liver and kidneys play an important role in the clearance of the drug, while the liver is also involved in the metabolism of riociguat to its active metabolite, M1 (unpublished data). Smoking also affects riociguat exposure, as the polycyclic aromatic hydrocarbons in cigarette smoke induce CYP1A1,18 the main enzyme responsible for riociguat metabolism.
To assess the pharmacokinetics (PK) of riociguat and M1 in patients with hepatic or renal impairment after a single dose of riociguat, four observational studies were conducted: two in patients with mild to moderate hepatic impairment,19,20 and two in patients with mild, moderate, or severe renal impairment.21,22 The reason for conducting two studies in each patient type was to determine the effect of renal and hepatic impairment on the PK of riociguat and M1 in the absence of smoking. At the time of conducting the first two studies, the effect of smoking on riociguat PK was unknown, and patients who were smokers were included in both studies. It was only after the studies had been completed that the effect of smoking on riociguat PK was determined, and it was found that smoking reduced riociguat and M1 exposure. Therefore, the studies in patients with renal and hepatic impairment were repeated in nonsmokers only.
The aim of the present analysis of data from these four studies was to characterize the PK of riociguat in patients with renal or hepatic impairment compared with that in healthy control groups and to evaluate possible covariates that influence the PK. The results would indicate whether renal or hepatic impairment might affect the required dosing of riociguat. An additional part of the analysis was to determine whether smoking status might affect riociguat dosing.
Methods
Study design
All four trials were single-center, nonrandomized, observational studies. Studies 1 and 2 enrolled patients with mild to severe renal impairment and age-, weight-, and sex-matched volunteers with normal renal function. Sample sizes were determined in accordance with US Food and Drug Administration guidelines for PK studies in patients with renal and hepatic impairment.23,24 Riociguat was given as a single oral dose of 1.0 mg (two 0.5-mg immediate-release tablets) in the morning under fasted conditions except for patients with severe renal impairment in study 1, who received a single 0.5-mg dose. Studies 3 and 4 were performed in patients with mild to moderate hepatic impairment with cirrhosis plus a cohort of healthy age-, weight-, and sex-matched volunteers. Riociguat was given as a single oral dose of 1.0 mg (two 0.5-mg immediate-release tablets) in the morning under fasted conditions.
Ethics
The protocols of the original studies were reviewed and approved by the Ethics Committee of the Schleswig-Holstein Medical Council (Bad Segeberg, Germany). The studies met all local legal and regulatory requirements and were conducted in accordance with the Declaration of Helsinki, the International Conference on Harmonization Good Clinical Practice Guideline, and German drug law. The protocol for subsequent PK modeling (which required no further patient interventions) was approved by the study sponsor.
All studies were conducted at a single center in Germany, and all laboratory determinations were performed by central laboratories. All participants in the four studies provided written informed consent.
Inclusion criteria
Participants in the four studies were aged 18–79 years of age, with a body mass index (BMI) of 18–32 (18–34 for those with hepatic impairment). Women of childbearing potential were permitted to enroll only if a pregnancy test was negative and they were using a combination of condoms with safe and highly effective contraception.
Participants in the two studies of liver impairment (Child-Pugh A or B) were white men or women with documented liver cirrhosis, confirmed by histopathology or ultrasound, and stable liver disease. Hepatic impairment was assessed at baseline using the Child-Pugh classification.25,26 Healthy controls were white men and women who were age- (±10 years), weight- (±10 kg of body weight), and sex matched to a participant with liver cirrhosis as far as possible.
For the renal impairment studies, creatinine clearance (CrCL) was determined 2–14 days before dosing and was classified as follows: normal, ≥80 mL/min; mild, 50 to <80 mL/min; moderate, 30 to <50 mL/min; and severe, <30 mL/min. Healthy controls were white men and women; mean age and body weight in the control and renal impairment groups were not allowed to vary by more than ±10 years and ±10 kg, respectively.
Exclusion criteria
Patients were excluded if they had participated in another clinical trial during the preceding 3 months (for a prior multidose study) or 1 month (for a prior single-dose study). In addition, smokers were excluded from studies 2 and 4.
Patients with hepatic impairment were excluded if they had clinical or biochemical evidence of hepatic decompensation and concomitant use of any medications other than those needed for the treatment of liver disease or related complications. Patients with renal impairment were excluded if they had unstable renal disease, acute renal failure, any organ transplant, or concomitant use of any medications other than those needed for the treatment of kidney disease or related complications. Further exclusion criteria regarding comorbidities are published elsewhere in this supplement.19,21 None of the subjects had findings on medical history or physical examination that were considered likely to interfere with the study objectives, and none of the concomitant medications during the study was considered likely to interfere with the study objectives.
PK sampling and analysis
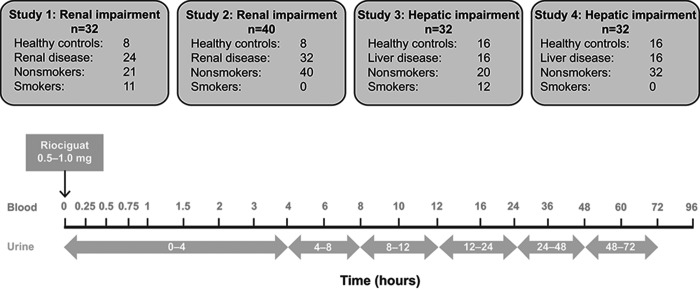
Blood samples for PK analysis were obtained before the dose and then at 0.25, 0.5, 0.75, 1, 1.5, 2, 3, 4, 6, 8, 10, 12, 16, 24, 36, 48, 60, 72, and 96 hours after administration of riociguat (Fig. 1). Unbound concentrations for riociguat and M1 in serum (as well as albumin and total protein) were determined for each patient in plasma samples at 2, 8, and 24 hours. Urine samples were collected at 0–4, 4–8, 8–12, 12–24, 24–48, and 48–72 hours for determination of riociguat and M1 (Fig. 1).
Figure 1.
Overview of the patient groups in the four studies and sampling scheme. Patients and healthy controls who fasted overnight received a single dose of riociguat 1.0 mg (0.5 mg for patients with severe renal impairment). Blood and urine samples were collected during the subsequent 96 hours.
Quantitative analyses of riociguat and M1 were determined by high-performance liquid chromatography and tandem mass spectrometry after protein precipitation (plasma) or dilution (urine). The analyses were performed in accordance with the 2001 Food and Drug Administration guideline on bioanalytical method validation.27 The calibration range of the procedure was from 0.5 µg/L (lower limit of quantification [LLOQ]) to 250 µg/L for plasma and from 10 µg/L (LLOQ) to 1,000 µg/L for urine. For the quantitative analysis in plasma, quality control (QC) samples in the concentration range 1.5–200 µg/L were determined with an accuracy of 98.2%–101.0% and a precision of 4.45%–5.50% for riociguat and 97.0%–99.0% and 4.49%–7.48%, respectively, for M1. For the quantitative analysis of urine, QC samples in the concentration range 30–800 µg/L were determined with an accuracy of 99.1%–103.0% and a precision of 4.88%–6.19% for riociguat and 98.0%–104.0% and 2.53%–8.87%, respectively, for M1. All samples were stored at ≤15°C and analyzed within 1 year after sampling. Stability data indicated that the analytes were stable for this time period. Determination of protein binding was performed in vitro using [14C]riociguat and [14C]M1, added to plasma samples at a concentration of 500 µg/L each.
Population PK modeling
All analyses were performed using the nonlinear mixed-effects modeling program NONMEM (ver. 7), on the basis of existing knowledge of riociguat PK, including its metabolism to M1. All subsequent statistics and graphics were made using SAS (ver. 8.2).
The population model consists of three submodels: the structural, statistical, and covariate models. The structural PK submodel describes the deterministic structure using fixed-effect parameters, while the statistical submodel accounts for variability using various levels of random effects, interindividual and interoccasion variability, and residual variability.
The covariate model investigated relationships between PK parameters and the following covariates: age, body weight, sex, CrCL, total protein, albumin, hematocrit, hemoglobin, pseudocholinesterase, bilirubin, prothrombin time (Quick test), Child-Pugh score and group, and fraction unbound. Model components or covariates were incorporated into the model if the likelihood ratio test showed significance (P = 0.01). The model component remained in the model when, after backward stripping from the full model, the likelihood ratio test showed significance (P = 0.001).
Residual variability was tested as being additive, proportional, or a combination of both. To reduce unexplained variability, the PK model structure was optimized with respect to the deterministic structure, interindividual variability, and residual variability.
Structural PK model
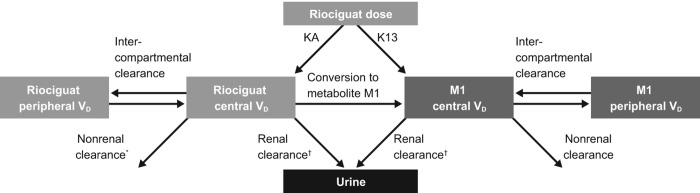
The model consisted of two-compartment models for riociguat and M1 (Fig. 2). The oral dose enters the central compartment with first-order kinetics after a lag time, and a small fraction is metabolized presystemically.
Figure 2.
Structural model consisting of two-compartment models for both riociguat and M1. The clearance of both riociguat and M1 is split into a renal component (filtration and excretion) and a nonrenal component, which for riociguat is further divided into conversion to M1 and a remaining nonrenal component. The oral dose enters the central compartment with first-order kinetics with a rate constant KA. A small fraction of the dose is metabolized presystemically, as described by the additional rate constant K13. Both absorption processes start after a common lag time. KA: absorption rate constant; K13: kinetic constant for the presystemic metabolism (<1%); M1: main metabolite of riociguat; VD: volume of distribution. *Other than conversion to M1; †filtration and secretion.
The clearances of parent and metabolite M1 were each split into a renal part (filtration and excretion) and a nonrenal part. Renal filtration was calculated as the product of the individual measured unbound fraction of the corresponding compound multiplied by the individual baseline CrCL. For riociguat, the nonrenal part was further divided into the conversion to M1 and a remaining metabolic (nonrenal) part.
Model validation
To investigate the validity of the established final model, a visual predictive check was conducted. Bayesian estimates for the PK parameters were generated using the population means and individual patient information by simulating 200 subproblems. Ninety percent prediction intervals were calculated and compared with the actual observations.
Results
Data used
An overview of each study population is given in Figure 1. Data from each of the two renal impairment studies were obtained from individuals with mild to severe renal impairment and age-matched men and women with normal renal function (total n = 72). Participants in the renal impairment studies were classified as having normal renal function (CrCL of ≥80 mL/min), mild renal impairment (CrCL of 50 to <80 mL/min), moderate renal impairment (CrCL of 30 to <50 mL/min), or severe renal impairment (CrCL of <30 mL/min; not receiving dialysis). Data from each of the two hepatic impairment studies were obtained from patients with mild to moderate hepatic impairment and healthy age-, weight-, and sex-matched individuals (total n = 64). The total number of PK samples is shown in Table 1. Distributions of continuous and categorical covariates in the patient populations of the studies are shown in Table 2.
Table 1.
Summary data used for pharmacokinetic analyses in studies including smokers (1 and 3) and studies excluding smokers (2 and 4)
| Samples, no. of subjects | Renal impairment | Hepatic impairment | Total |
|---|---|---|---|
| Study 1 | Study 3 | ||
| Riociguat 0.5 mg | 249 | … | 249 |
| Riociguat 1.0 mg | 914 | 1,245 | 2,159 |
| Total | 1,163 | 1,245 | 2,408 |
| Study 2 | Study 4 | ||
| Riociguat | |||
| Plasma | 650 | 527 | 1,177 |
| Urine | 171 | 148 | 319 |
| Metabolite M1 | |||
| Plasma | 641 | 517 | 1,158 |
| Urine | 141 | 130 | 271 |
Table 2.
Continuous and categorical covariates used in the population pharmacokinetic analysis
| Studies 1 and 3 (with smokers; n = 64) | Studies 2 and 4 (with nonsmokers; n = 72) | |||
|---|---|---|---|---|
| Parameter | Mean (SD) | Median (range) | Mean (SD) | Median (range) |
| Continuous covariates | ||||
| Age, years | 57.5 (9.5) | 58.5 (35–74) | 60.0 (10.3) | 61.0 (36–78) |
| Weight, kg | 82.0 (12.6) | 83.3 (56–108.3) | 83.1 (13.9) | 86.5 (54.2–117) |
| Baseline level | ||||
| Albumin, g/dL | 4.0 (0.4) | 4.0 (3.2–5.1) | 4.0 (0.4) | 4.1 (3.1–4.9) |
| Bilirubin, mg/dL | 0.7 (1.0) | 0.5 (0.2–7.2) | 0.6 (0.9) | 0.4 (0.1–6.1) |
| Hemoglobin, g/dL | … | … | 13.0 (2.0) | 13.4 (7.7–17.4) |
| Hematocrit, % | … | … | 39.7 (5.4) | 40.8 (25.1–50.3) |
| Protein, g/dL | 6.9 (0.6) | 6.8 (5.7–8.2) | 6.8 (0.6) | 6.7 (5.7–8.7) |
| Derived CrCL,a mL/min | 74.1 (27.4) | 75.1 (17.9–125.5) | 69.4 (25.5) | 72.7 (7.0–129.3) |
| Measured baseline CrCL,b mL/min | 76.6 (36.1) | 77.6 (10–172.3) | 79.9 (45.9) | 73.5 (8.7–274.3) |
| Riociguat unbound fraction, % | 3.3 (1.0) | 3.2 (1.7–9.2) | 4.0 (0.9) | 4.0 (2.3–8.1) |
| M1 unbound fraction, % | 3.1 (1.0) | 2.9 (1.8–8.5) | 3.3 (0.9) | 3.2 (2.0–8.7) |
| Riociguat free concentration,c %·mL/min | 254.2 (161.8) | 238.7 (37.7–1,067.1) | 252.0 (144.4) | 209.4 (45.8–969.2) |
| M1 free concentration,d %·mL/min | 238.0 (157.0) | 215.8 (35.8–982.7) | 304.6 (178.4) | 265.2 (49.3–1,224.3) |
| Categorical covariates, no. | ||||
| Sex | ||||
| Male | 19 | 20 | 28 | 22 |
| Female | 13 | 12 | 12 | 10 |
| Smoking status | ||||
| Smokers | 11 | 12 | 0 | 0 |
| Nonsmokers | 21 | 20 | 40 | 32 |
CrCL: creatinine clearance; M1: main metabolite of riociguat.
CrCL calculated from serum creatinine concentrations at baseline.
CrCL measured using plasma and urine concentrations at baseline.
Riociguat unbound fraction × measured creatinine clearance.
M1 unbound fraction × measured creatinine clearance.
Population PK modeling
Base model
As described above (see “Structural PK model”), the base model consisted of two-compartment models for riociguat and M1. The oral dose entered the central compartment with first-order kinetics after a lag time and a small fraction was metabolized presystemically, while clearances of each compound were split into renal and nonrenal components.
Population estimates of the parameters for the base model are shown in Table 3. The dominant clearance route for riociguat was metabolism to M1. Populations that included smokers (studies 1 and 3) showed higher clearance by metabolism to M1 than those without smokers (studies 2 and 4). Clearance resulting from renal secretion of riociguat and metabolite M1 showed high interindividual variability (coefficient of variation of ∼80% or higher) in populations with or without smokers (Table 3). The nonrenal clearance of M1, which is the dominant clearance path for this substance, had a lower variability (coefficient of variation of ∼37.0%–38.5%).
Table 3.
Population estimates for the base pharmacokinetic model
| Studies 1 and 3 (with smokers) | Studies 2 and 4 (with nonsmokers) | |||
|---|---|---|---|---|
| Parameter | Mean estimate | IIVa | Mean estimate | IIVa |
| Riociguat | ||||
| Absorption rate, 1/h | 3 fixb | 68.7 | 3 fixb | 66.7 |
| Presystemic metabolism, 1/h | 0.0122 | NA | 0.00787 | NA |
| Clearance by renal secretion, L/h | 0.106 | 78.5 | 0.06985 | 90.9 |
| Clearance by metabolism to M1, L/h | 2.32 | 98.0 | 1.24 | 74.4 |
| Clearance by nonrenal mechanisms,c L/h | 0.630 | 82.3 | 0.424 | 61.1 |
| Central volume of distribution, L/kg | 0.271 | 30.3 | 0.238 | 22.1 |
| Intercompartmental clearance, L/h | 2.23 | NA | 2.54 | NA |
| Peripheral volume of distribution, L/kg | 0.114 | 37.1 | 0.132 | 41.7 |
| Metabolite M1 | ||||
| Clearance by renal secretion, L/h | 0.345 | 81.4 | 0.266 | 79.9 |
| Nonrenal clearance, L/h | 1.74 | 37.1 | 1.71 | 38.5 |
| Central volume of distribution, L/kg | 0.130 | 29.6 | 0.108 | 31.6 |
| Intercompartmental clearance, L/h | 10.3 | NA | 12.9 | NA |
| Peripheral volume of distribution, L/kg | 0.376 | 26.3 | 0.449 | 16.6 |
| Absorption lag time, hours | 0.243 | 35.1 | 0.221 | 36.3 |
| Proportional residual error (plasma), % | 19.4 | … | 17.7 | … |
| Proportional residual error (urine), % | 34.9 | … | 41.2 | … |
IIV: interindividual variability; M1: main metabolite of riociguat; NA: not available.
Calculated as the square root of the variance (which is approximately equivalent to the coefficient of variation [%]).
Absorption rate constant (KA) was fixed to achieve a stable model that would allow precise estimates of IIV. The KA value of 3 was chosen because this was the estimated KA value during early steps in model development.
Other than conversion to M1.
Final model
The final model was derived on the basis of covariate analyses; covariates with relevant influence on the model were included, and their relevance was retested in the backward elimination step of the model development. The final model was derived by applying the following covariates: the unbound fraction on the nonrenal clearance of riociguat and the central volume of distribution of riociguat, the derived CrCL at baseline on the nonrenal clearance of M1, and the CrCL measured at baseline and at follow-up on the nonrenal clearance of M1. Incorporation of the covariates reduced the coefficient of variation for the nonrenal clearance of riociguat (excluding conversion to M1) from 61% to 50%. The coefficient of variation for the renal excretion of M1 was reduced from 80% to 57%.
Goodness-of-fit plots are shown in Figure 3. The results of the visual predictive check for riociguat and M1 confirm the validity of the model, with the majority of observed values falling within the 90% prediction interval (Fig. 4).
Figure 3.
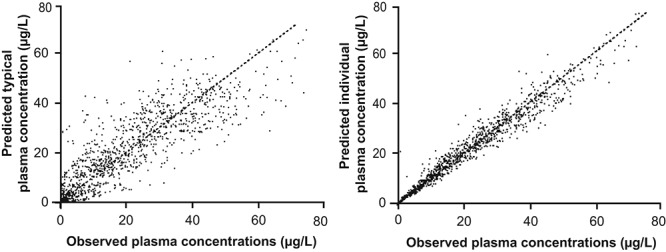
Goodness-of-fit plots for the final model showing the relationship between predicted typical and individual plasma concentrations and observed plasma concentrations.
Figure 4.
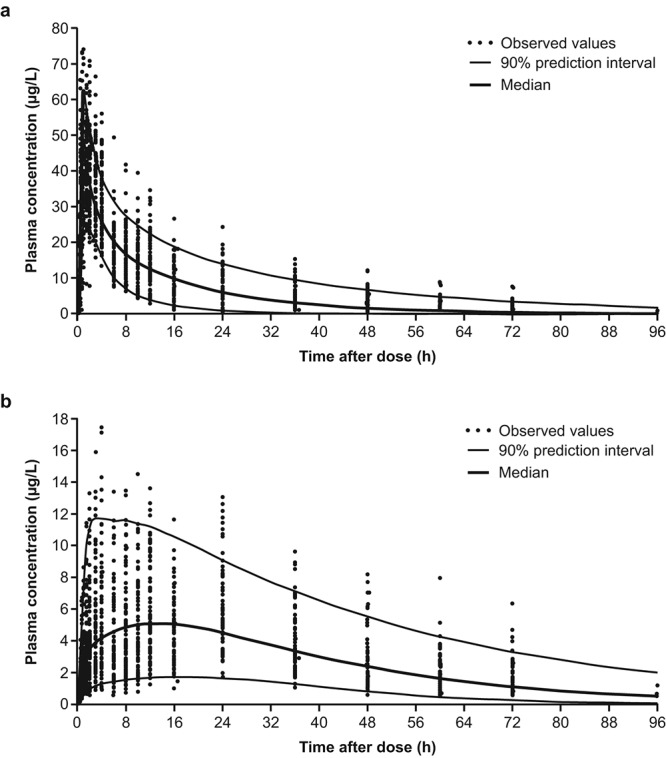
Visual predictive check for riociguat (a) and M1 (b; all patients). Individual empirical Bayesian estimates for the pharmacokinetic parameters were estimated using the population means and individual patient information by simulating n = 200 subproblems; 90% prediction intervals were calculated and compared to the actual observations.
Population estimates of the final model parameters are shown in Table 4. The oral dose enters the central compartment with first-order kinetics (rate constant: 3 L/h) after a lag time of 0.22 hours. A small fraction of the dose (<1%) is metabolized to M1 presystemically, as described by the additional rate constant K13 (Fig. 2).
Table 4.
Population estimates for the final pharmacokinetic model
| Parameter | Mean estimate | Relative standard error, %a | IIV, %b | Relative standard error, %a |
|---|---|---|---|---|
| Riociguat | ||||
| Absorption rate, 1/h | 3 fixc | NA | 66.0 | 21.8 |
| Presystemic metabolism, 1/h | 0.00906 | 29.8 | NA | NA |
| Clearance by renal secretion, L/h | 0.0685 | 15.5 | 90.1 | 26.1 |
| Clearance by metabolism to M1, L/h | 1.20 | 9.8 | 75.8 | 14.0 |
| Clearance by nonrenal mechanisms,d L/h | 0.338 | 14.7 | 50.1 | 24.2 |
| Change in nonrenal clearancec per unit of riociguat (unbound fraction), difference from the median (3.2%)e | 51.9 | 22.7 | NA | NA |
| Central volume of distribution per kg of body weight, L/kg | 0.214 | 4.4 | 18.8 | 19.7 |
| Change in central volume of distribution per unit of riociguat (unbound fraction), difference from the median (3.2%)f | 15.5 | 27.1 | NA | NA |
| Intercompartmental clearance, L/h | 2.57 | 9.5 | NA | NA |
| Peripheral volume of distribution per kg of body weight, L/kg | 0.132 | 6.3 | 41.8 | 22.9 |
| Metabolite M1 | ||||
| Clearance by renal secretion per unit of CrCL at baseline (mL/min) and per unit of M1 (unbound fraction) at baseline, % (L/h/[mL/min·%])g | 0.00119 | 8.4 | 57.0 | 21.4 |
| Clearance by nonrenal mechanisms, L/h | 1.76 | 5.9 | 30.5 | 30.0 |
| Change in nonrenal clearance of M1 per unit of derived CrCL,h difference from the median (75 mL/min)i | 0.744 | 22.6 | NA | NA |
| Central volume of distribution per kg of body weight | 0.108 | 8.9 | 32.7 | 24.1 |
| Intercompartmental clearance, L/h | 12.2 | 6.6 | NA | NA |
| Peripheral volume of distribution per kg of body weight, L/kg | 0.428 | 4.0 | … | … |
| Absorption lag time, hours | 0.220 | 4.5 | 36.3 | 21.4 |
| Proportional residual error, % | ||||
| Plasma | 17.9 | 6.5 | NA | NA |
| Urine | 41.4 | 9.1 | NA | NA |
CrCL: creatinine clearance; IIV: interindividual variability; NA: not available.
Expressed as a percentage of the estimate.
Calculated as the square root of the variance, which is approximately equal to the coefficient of variation (%).
Absorption rate constant (KA) was fixed to achieve a stable model that would allow precise estimates of IIV. The KA value of 3 was chosen because this was the estimated KA value during early steps in model development.
Other than conversion to M1.
Typical value = 0.338 (θ4) × (1 + 0.519 [θ17]) × (riociguat unbound fraction − median riociguat unbound fraction).
Typical value = 0.214 (θ5) × body weight × (1 + 0.154 [θ16]) × (riociguat unbound fraction − median riociguat unbound fraction).
Typical value = 0.001 (θ8) × M1 unbound fraction × measured CrCL (measured using plasma and urine concentrations at baseline).
CrCL was calculated from serum creatinine concentration at baseline.
Typical value = 1.76 (θ9) × (1 + 0.007 [θ15]) × (derived CrCL − median derived CrCL).
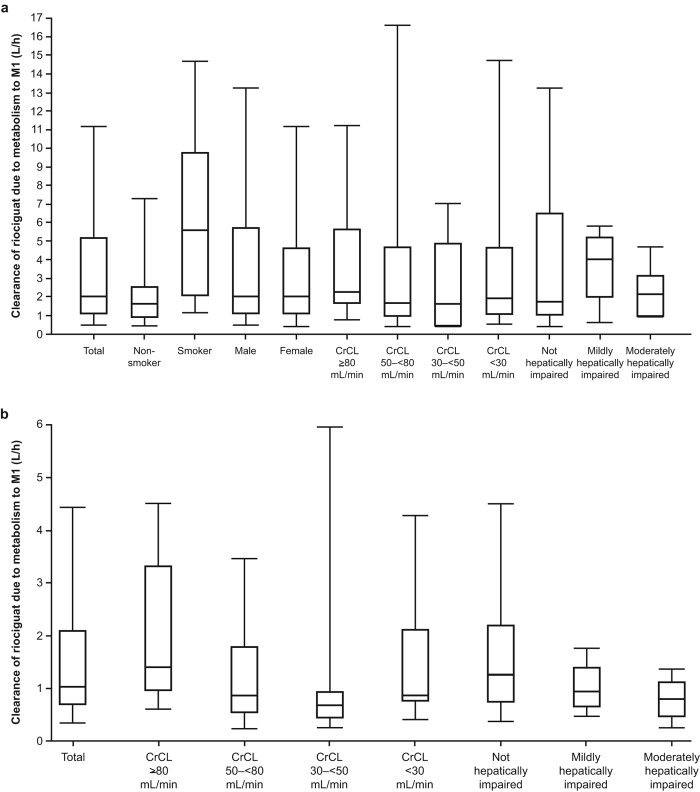
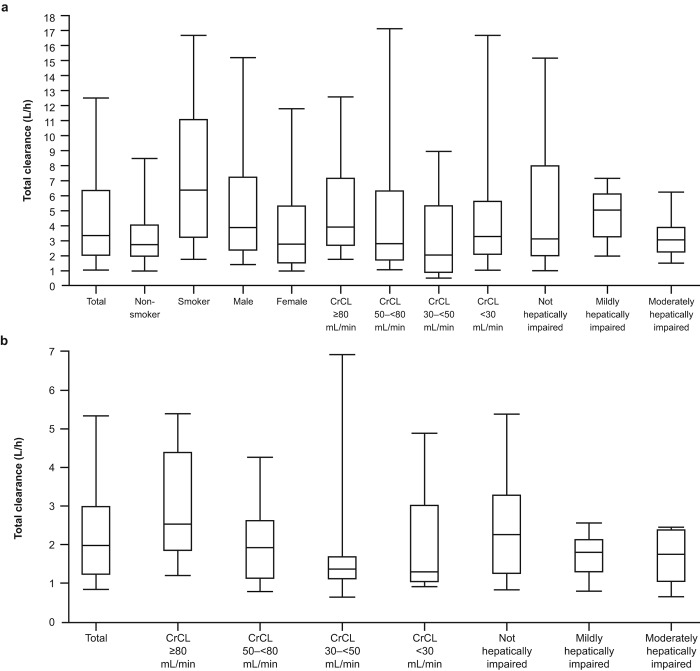
On the basis of these results, a CrCL of 80 mL/min and a typical unbound fraction of 3% would lead to an estimated clearance due to renal filtration of 2.4 mL/min, or about 0.15 L/h, for both riociguat and M1. Elimination of riociguat is dominated by conversion to M1, with a typical value of 1.2 L/h; individual values of rate of metabolism to M1 show high variability, and the unexplained variability of this clearance contribution is high. The rates of clearance of riociguat by metabolism to M1 in the studies including smokers and nonsmokers are shown in Figure 5. Corresponding median total clearance rates are shown in Figure 6.
Figure 5.
Clearance of riociguat by metabolism to M1 in studies 1 and 3 with smokers (a) and studies 2 and 4 with nonsmokers (b). Boxes represent 25th–75th percentiles, horizontal lines represent medians, and error bars represent 5th–95th percentiles. CrCL: creatinine clearance; M1: main metabolite of riociguat.
Figure 6.
Total clearance of riociguat in studies 1 and 3 with smokers (a) and studies 2 and 4 with nonsmokers (b). Boxes represent 25th–75th percentiles, horizontal lines represent medians, and error bars represent 5th–95th percentiles. CrCL: creatinine clearance.
Riociguat clearance by nonrenal mechanisms (other than conversion to M1) in the final model is proportional to the unbound fraction and has a typical value of 0.34 L/h (Table 4). Similar values for nonrenal clearance of riociguat were estimated in all groups included in studies 2 and 4 (i.e., renal and hepatic impairment and healthy individuals; data not shown). No significant influence of biomarkers for hepatic function (e.g., albumin, bilirubin) or Child-Pugh classification could be established on clearance of riociguat or M1. The mean total clearance of riociguat in nonsmokers (1.912 L/h) is the sum of mean renal filtration (0.174 L/h), mean renal secretion (0.068 L/h), mean nonrenal clearance (0.496 L/h), and mean metabolism to M1 (1.2 L/h; Table 5).
Table 5.
Effects of smoking on the population pharmacokinetics of riociguat
| Mean estimate | |||
|---|---|---|---|
| Parameter | Studies 1 and 3 (with smokers) | Studies 2 and 4 (with nonsmokers) | All studies |
| Clearance by renal filtration, L/h | 0.148 | 0.174 | 0.159 |
| Clearance by renal secretion, L/h | 0.117 | 0.068 | 0.093 |
| Clearance by metabolism to M1, L/h | |||
| Nonsmokers | 1.60 | 1.20 | 1.23 |
| Smokers | 4.48 | … | 4.329 |
| Nonrenal clearance, L/h | Male: 0.979 Female: 0.287 |
0.496 | Male: 0.798 Female: 0.372 |
| Total clearance, L/h | |||
| Nonsmokers | Male: 2.845 Female: 2.154 |
1.912 | Male: 2.281 Female: 1.855 |
| Smokers | Male: 5.725 Female: 5.040 |
… | Male: 5.380 Female: 4.954 |
M1: main metabolite of riociguat.
The relationship between total clearance of riociguat and CrCL by renal function is shown in Figure 7. Patients with moderate or severe renal impairment tended to have lower total clearance. Total clearance in patients with mild to moderate hepatic impairment was comparable to that in healthy individuals (Fig. 8). The healthy group included individuals with a higher clearance rate compared with the other studied populations (Figs. 7, 8). No parameters could be identified to explain the higher total clearance rate in these patients, which is mainly driven by the metabolism of riociguat to M1.
Figure 7.
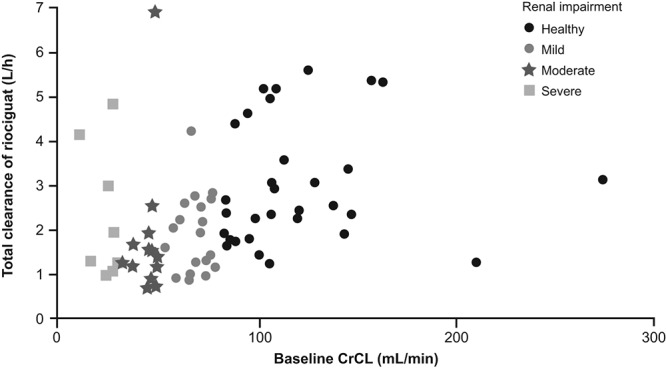
Relationship between total clearance of riociguat versus creatinine clearance (CrCL) categorized by renal classification. Data are shown for all subjects included in the renal and hepatic studies that excluded smokers (studies 2 and 4). Symbols show renal classification.
Figure 8.
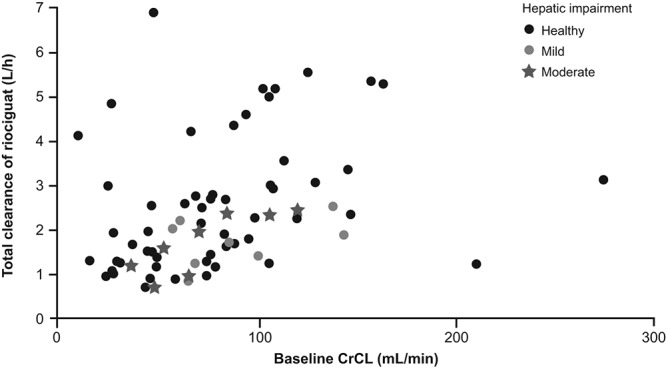
Relationship between total clearance of riociguat versus creatinine clearance (CrCL) categorized by hepatic classification. Data are shown for all subjects included in the renal and hepatic studies that excluded smokers (studies 2 and 4). Symbols show hepatic classification.
Effect of smoking
When data were pooled from studies 1 and 3 and across all four studies, there was no relevant change in the estimates of the PK parameters in the three models. The sex dependency observed in studies 1 and 3 was not seen in studies 2 and 4 and does not contribute to a reduction of the variability in the combined model. The mean total clearance in all studies excluding smokers was similar. A significant increase in the metabolism of riociguat to M1 was observed in smokers compared with nonsmokers in all studies (Table 5).
Discussion
The liver and kidneys play important roles in the metabolism and clearance of riociguat. It is possible, therefore, that impairment of hepatic or renal function could alter the PK of the drug and its major metabolite, M1. In this analysis, we investigated the potential impact of renal and hepatic impairment on riociguat using a population PK modeling approach.
In this model, riociguat clearance is dominated by its metabolism to M1, which shows high interindividual variability independent of renal or hepatic status. Renal clearance of riociguat is mainly determined by renal filtration, as measured by CrCL. There is thus a clear effect of moderate and severe renal impairment on renal clearance. Because of the limited contribution of renal clearance to total drug clearance, however, the influence of renal impairment on total clearance is weak, and alterations in drug exposure are expected to be moderate.
Results from this population PK model also show no indication that biomarkers for hepatic function (e.g., albumin, bilirubin) or Child-Pugh classification significantly influence any clearance contributions for riociguat or M1. However, it is important to note that these data relate to the current model only, which uses mixed patients with renal and hepatic impairment, and not results from individual PK studies. In the individual PK studies, results for nonsmokers showed that exposure to riociguat (the dose-normalized area under the plasma concentration time curve) was increased by 51% in patients with Child-Pugh B hepatic impairment compared with healthy controls: this difference was not statistically significant.19 In patients with Child-Pugh A impairment, exposure was increased by 35%, which is within the range of normal variability. In nonsmoking individuals with mild, moderate, or severe renal impairment, riociguat exposure was increased by 53%, 139%, and 54%, respectively.19
The metabolism of riociguat to M1 was increased in smokers compared with that in nonsmokers, which can be explained by the difference in enzymatic activity of cytochrome CYP1A1 in these patients, as CYP1A1 is the decisive enzyme of riociguat metabolism16,17 and is induced by polycyclic aromatic hydrocarbons in cigarette smoke.18
In conclusion, in this population PK analysis the PK of riociguat was described well by a two-compartment model. Total riociguat clearance was dominated by its metabolism to M1, and renal clearance was mainly determined by filtration. Renal impairment reduced riociguat and M1 renal clearance, but due to the limited role of renal clearance in the total clearance of riociguat, the model predicted moderate effects of renal impairment on drug exposure. This was confirmed by the individual PK studies in which riociguat exposure was increased by 53%–139% in the presence of renal dysfunction. Smoking increased the metabolism of riociguat to M1.
The results described here are reflected in the advice given in the product label for riociguat, which states that riociguat has not been studied in patients with severe (Child-Pugh C) hepatic impairment.16,17 Treatment is therefore not recommended for these patients (and is contraindicated in some countries). Patients with moderate hepatic impairment (Child-Pugh B) have increased riociguat exposure, and therefore particular care should be exercised during individual dose adjustment in these patients. No dose adjustment is required for patients with mild hepatic impairment (Child-Pugh A).
With respect to renal impairment, the product label for riociguat states that data are limited in severe impairment (CrCL of <30 mL/min), and there are no data on patients receiving dialysis.16,17 Riociguat is therefore not recommended in the United States in patients with CrCL of <15 mL/min, and in Europe riociguat is not recommended in patients with CrCL of <30 mL/min. Patients with moderate renal impairment (CrCL of 30 to <50 mL/min) have increased riociguat exposure, and particular care should be exercised during individual dose titration in these patients. There are no specific recommendations for dose titration or adjustment in patients with mild renal impairment.
Plasma concentrations of riociguat are reduced in smokers compared with those in nonsmokers, so dose adjustment may be necessary in patients who start or stop smoking during treatment with riociguat.
Acknowledgments
We gratefully acknowledge Dagmar Klein and Matthias Frede (Clinical Sciences, Pharmacometrics, Bayer HealthCare Pharmaceuticals, Wuppertal, Germany) for the skilful data analysis and their contributions to the study.
Source of Support: The study was funded by a research grant from Bayer Pharma (Wuppertal, Germany). Medical writing support was provided by Adelphi Communications and was funded by Bayer Pharma.
Conflict of Interest: All authors are employees of Bayer Pharma.
References
- 1.Klinger JR, Abman SH, Gladwin MT. Nitric oxide deficiency and endothelial dysfunction in pulmonary arterial hypertension. Am J Respir Crit Care Med 2013;188(6):639–646. [DOI] [PubMed]
- 2.Stasch JP, Evgenov OV. Soluble guanylate cyclase stimulators in pulmonary hypertension. Handb Exp Pharmacol 2013;218:279–313. [DOI] [PubMed]
- 3.Stasch JP, Pacher P, Evgenov OV. Soluble guanylate cyclase as an emerging therapeutic target in cardiopulmonary disease. Circulation 2011;123(20):2263–2273. [DOI] [PMC free article] [PubMed]
- 4.Schermuly RT, Stasch JP, Pullamsetti SS, Middendorff R, Muller D, Schluter KD, Dingendorf A, et al. Expression and function of soluble guanylate cyclase in pulmonary arterial hypertension. Eur Respir J 2008;32(4):881–891. [DOI] [PubMed]
- 5.Follmann M, Griebenow N, Hahn MG, Hartung I, Mais FJ, Mittendorf J, Schafer M, et al. The chemistry and biology of soluble guanylate cyclase stimulators and activators. Angew Chem Int Ed Engl 2013;52(36):9442–9462. [DOI] [PubMed]
- 6.Dumitrascu R, Weissmann N, Ghofrani HA, Dony E, Beuerlein K, Schmidt H, Stasch JP, et al. Activation of soluble guanylate cyclase reverses experimental pulmonary hypertension and vascular remodeling. Circulation 2006;113(2):286–295. [DOI] [PubMed]
- 7.Geschka S, Kretschmer A, Sharkovska Y, Evgenov OV, Lawrenz B, Hucke A, Hocher B, Stasch JP. Soluble guanylate cyclase stimulation prevents fibrotic tissue remodeling and improves survival in salt-sensitive Dahl rats. PLoS ONE 2011;6(7):e21853. [DOI] [PMC free article] [PubMed]
- 8.Lang M, Kojonazarov B, Tian X, Kalymbetov A, Weissmann N, Grimminger F, Kretschmer A, et al. The soluble guanylate cyclase stimulator riociguat ameliorates pulmonary hypertension induced by hypoxia and SU5416 in rats. PLoS ONE 2012;7(8):e43433. [DOI] [PMC free article] [PubMed]
- 9.Sharkovska Y, Kalk P, Lawrenz B, Godes M, Hoffmann LS, Wellkisch K, Geschka S, et al. Nitric oxide–independent stimulation of soluble guanylate cyclase reduces organ damage in experimental low-renin and high-renin models. J Hypertens 2010;28(8):1666–1675. [DOI] [PubMed]
- 10.Evgenov OV, Zou L, Zhang M, Mino-Kenudson M, Mark EJ, Buys ES, Li Y, et al. Stimulation of soluble guanylate cyclase attenuates bleomycin-induced pulmonary fibrosis in mice. Am J Respir Crit Care Med 2011;183:A2715.
- 11.Stasch JP, Hobbs AJ. NO-independent, haem-dependent soluble guanylate cyclase stimulators. Handb Exp Pharmacol 2009;191:277–308. [DOI] [PubMed]
- 12.Methner C, Buonincontri G, Hu CH, Vujic A, Kretschmer A, Sawiak S, Carpenter A, Stasch JP, Krieg T. Riociguat reduces infarct size and post-infarct heart failure in mouse hearts: insights from MRI/PET imaging. PLoS ONE 2013;8(12):e83910. [DOI] [PMC free article] [PubMed]
- 13.Ghofrani HA, D’Armini AM, Grimminger F, Hoeper MM, Jansa P, Kim NH, Mayer E, et al. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. N Engl J Med 2013;369(4):319–329. [DOI] [PubMed]
- 14.Ghofrani HA, Galiè N, Grimminger F, Grünig E, Humbert M, Jing ZC, Keogh AM, et al. Riociguat for the treatment of pulmonary arterial hypertension. N Engl J Med 2013;369(4):330–340. [DOI] [PubMed]
- 15.Bayer. Adempas [product monograph]. Toronto: Bayer, 2014.
- 16.European Medicines Agency. Annex I: summary of product characteristics [Adempas (riociguat tablets)]. Published March 27, 2014. Accessed July 21, 2015. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002737/WC500165034.pdf.
- 17.US Food and Drug Administration. Highlights of US prescribing information [Adempas (riociguat) tablets]. Published October 8, 2013. Accessed July 21, 2015. http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/204819s000lbl.pdf.
- 18.Zevin S, Benowitz NL. Drug interactions with tobacco smoking: an update. Clin Pharmacokinet 1999;36(6):425–438. [DOI] [PubMed]
- 19.Frey R, Becker C, Unger S, Schmidt A, Wensing G, Mück W. Assessment of the effects of hepatic impairment and smoking on the pharmacokinetics of a single oral dose of the soluble guanylate cyclase stimulator riociguat (BAY 63-2521). Pulm Circ 2016;6(S1):S5–S14. [DOI] [PMC free article] [PubMed]
- 20.Frey R, Becker C, Unger S, Schmidt A, Wensing G, Mueck W. Pharmacokinetics of the soluble guanylate cyclase stimulator riociguat in individuals with hepatic impairment. BMC Pharmacol Toxicol 2013;14(suppl. 1):P21.
- 21.Frey R, Becker C, Unger S, Schmidt A, Wensing G, Mück W. Assessment of the effects of renal impairment and smoking on the pharmacokinetics of a single oral dose of the soluble guanylate cyclase stimulator riociguat (BAY 63-2521). Pulm Circ 2016;6(S1):S15–S26. [DOI] [PMC free article] [PubMed]
- 22.Frey R, Becker C, Unger S, Schmidt A, Wensing G, Mueck W. Pharmacokinetics of the soluble guanylate cyclase stimulator riociguat in individuals with renal impairment. BMC Pharmacol Toxicol 2013;14(suppl. 1):P22.
- 23.US Food and Drug Administration. Guidance for industry: pharmacokinetics in patients with impaired hepatic function—study design, data analysis, and impact on dosing and labeling. Published May 2003. Accessed December 10, 2015. http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm072123.pdf.
- 24.US Food and Drug Administration. Guidance for industry: pharmacokinetics in patients with impaired renal function—study design, data analysis, and impact on dosing and labeling. Published March 2010. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM204959.pdf.
- 25.Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg 1973;60(8):646–649. [DOI] [PubMed]
- 26.Child CG, Turcotte J. The liver and portal hypertension. In: Child CG, ed. Surgery and portal hypertension. Philadelphia: WB Saunders, 1964:50–58.
- 27.US Food and Drug Administration. Guidance for industry: bioanalytical method validation. Published May 2001. Accessed September 25, 2015. http://www.fda.gov/downloads/Drugs/.../Guidances/ucm070107.pdf.