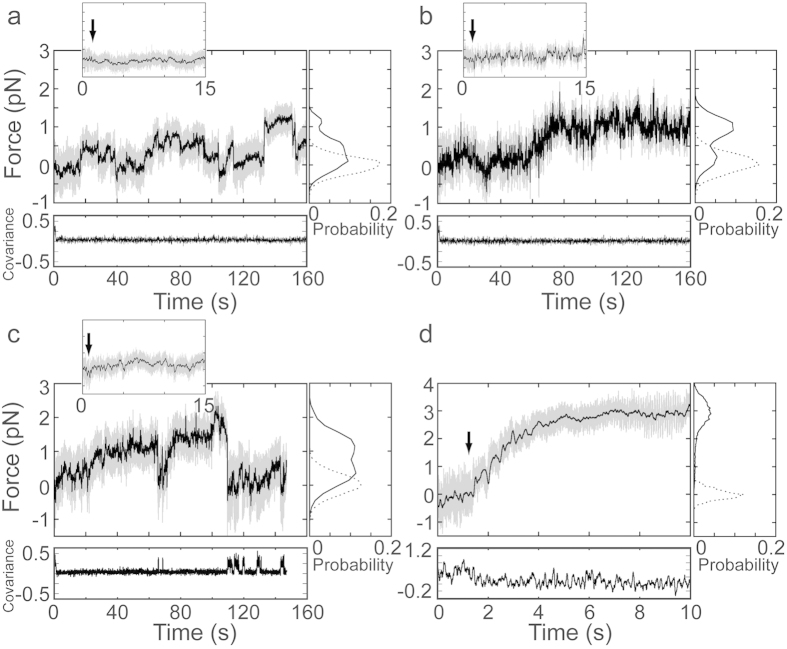
Figure 4. Force developed on actin filaments by ensembles of Myo1c molecules on lipid and solid substrates (1mM ATP).
(Large panels) Force on actin dumbbells pulled by Myo1c molecules bound to lipid coated spherical pedestals in the presence of (a) 15 nM and (b) 150 nM Myo1c. Actin dumbbells pulled by Myo1c3IQ molecules anchored via biotin-streptavidin linker on nitrocellulose-coated spherical pedestals in the presence of (c) 18 nM and (d) 360 nM Myo1c. Unfiltered (gray) and smoothed (black) force traces of the beads attached to the barbed-end of the actin filaments are shown. (Insets) Expanded views of the first 15 s of the force traces, with the black arrows indicating the instant of attachment of pedestal-bound Myo1c molecules to the actin filament. (Right subpanels) Probability histograms of the force before the initial attachment (dashed lines) and after the initial attachment (solid lines). (Lower subpanels) Covariance traces (×500) of the two laser-trapped beads. Low covariance values indicate attachment of the actin filament to pedestal-bound Myo1c molecules, while high covariance values indicate detachment.