Abstract
Background
Myocardial H2 receptor activation may promote cardiac fibrosis and apoptosis in preclinical models and histamine H2 receptor antagonist (H2RA) use may improve symptoms in participants with heart failure (HF); however, relationships between H2RA use, incident HF, and longitudinal change in left ventricular (LV) morphology are not known.
Objectives
We sought to determine whether H2RA use is associated with incident HF and change in LV morphology over time.
Methods
This study included 6,378 men and women from MESA (Multi-Ethnic Study of Atherosclerosis), a multicenter prospective observational cohort of participants without cardiovascular disease at baseline. Cox proportional hazards were used to estimate the association between H2RA use and incident HF in adjusted models. In participants with cardiac magnetic resonance imaging (CMR), associations between H2RA use, baseline LV morphology (n = 4,691), and longitudinal change in the LV (n = 2,806) were estimated using linear regression.
Results
H2RAs were used by 313 participants but not by the other 6,065 individuals. During a median follow-up of 11.2 years, 236 participants developed HF. In adjusted models, baseline H2RA use relative to nonuse was associated with 62% lower risk for incident HF (p = 0.02). H2RA use was associated with preserved stroke volume, LV end-diastolic volume, and mass/volume ratio as measured by CMR over approximately 10 years (all p < 0.05). There were no associations between H2RA use and LV mass or ejection fraction.
Conclusions
H2RA use was associated with reduced risk for incident HF. Changes in left heart morphology over time suggest less age-related change in H2RA users. These associations suggest histamine signaling may be important in the pathogenesis of HF.
Keywords: cardiac magnetic resonance imaging, heart failure, prevention
Histamine H2 receptor antagonists (H2RAs) are commonly used to treat gastroesophageal reflux disease (1) and have a relatively strong safety profile. Beyond their use for symptomatic control of gastroesophageal reflux, H2RAs also may have a role in cardiac disease. Similar to beta receptors, H2 receptors activate stimulatory G-proteins in the myocardium (2,3). Blockade of histamine receptors or histamine release can prevent heart failure (HF) in rabbits exposed to doxorubicin and dogs with pacemaker-driven tachycardia (4-6). Relative to mice with an intact H2 receptor, mice with the H2 receptor knock-out had improved cardiac function and developed less fibrosis when subjected to aortic banding (7). Additionally, H2 receptor activation may increase mitochondrial permeability in cardiac myocytes and myocardial susceptibility to stress (8).
In men and women with HF and reduced ejection fraction (HFrEF), H2RA use is associated with smaller left ventricular (LV) volumes and less severe symptoms; we have previously shown H2RA use is associated with lower right ventricular mass and smaller volumes (9,10). Alongside the observations in animals, these data suggest histamine signaling may be relevant to the pathogenesis of human heart failure.
This study examined associations between H2RA use and incident HF in a multiethnic cohort of adults free of clinical cardiovascular disease (CVD) at baseline. This study further evaluated associations between H2RA use, LV morphology, and change in LV morphology over time. The study investigators hypothesized that H2RA use would be associated with a reduced risk for heart failure.
Methods
MESA (Multi-Ethnic Study of Atherosclerosis) is a prospective cohort study designed to investigate subclinical CVD (11). From 2000 to 2002, MESA recruited participants aged 45 to 84 years old from 6 U.S. communities. Evaluation included 5 MESA examinations over approximately 10 years. Exclusion criteria included clinical CVD (physician-diagnosed myocardial infarction, stroke, transient ischemic attack, HF, angina, current atrial fibrillation, any cardiovascular procedure), weight >136 kg, pregnancy, or impediment to long-term participation. Institutional review boards of participating institutions approved the MESA protocols. All participants provided informed consent.
Cardiac magnetic resonance imaging (CMR) was obtained at the baseline examination for the majority of MESA participants. In a subset of these participants, CMR was repeated approximately 10 years later (12). The CMR protocol and interpretation of LV parameters in MESA have been previously described and are included in the Online Appendix (13,14).
Medication Use
A validated medication inventory was used to assess medication use (10,15). Participants were asked to bring all medications used during the 2 weeks prior to a MESA examination. Participants were considered H2RA users in primary analyses if they used prescription or over-the-counter H2RAs at the baseline MESA examination. Other baseline medication use considered for confounding included proton pump inhibitors, angiotensin-converting enzyme inhibitors (± diuretic), angiotensin II receptor blockers (± diuretic), beta-blockers (± diuretic), any diuretic alone (including potassium-sparing diuretics), oral steroids, nonsteroidal anti-inflammatory drugs (NSAIDs) (including aspirin, COX-2 inhibitors, and other NSAIDs), digoxin, and leukotriene antagonists.
Ascertainment of Events
Full details of event ascertainment and definition are available in MESA's manual of procedures (MOP) (16). Briefly, clinical outcomes were assessed at MESA study examinations and by telephone interview every 9 to 12 months. Records were obtained for approximately 99% of hospitalizations and 97% of outpatient cardiovascular diagnostic encounters through the end of calendar year 2012. Incident HF required symptoms of heart failure, a physician diagnosis of HF, and another objective feature of heart failure (dilated or poor LV function, pulmonary edema by chest radiograph, treatment, or evidence of diastolic dysfunction). Two physicians from the MESA events committee independently reviewed all medical records for classification and dating of events. If reviewers disagreed, they adjudicated differences. If disagreement persisted, the full events committee made the final classification.
Statistical Analysis
The methods of Kaplan and Meier were used to estimate unadjusted associations and Cox proportional hazards to estimate adjusted associations of H2RA use at the baseline exam with incident HF. Covariates were assessed at the initial MESA exam, described in MESA's MOP, and chosen a priori (16). In limited models, we adjusted for age, sex, race, height, weight, and study site. In adjusted models, we included participants' education and cardiovascular risk factors including intentional exercise, smoking status, pack-years of smoking, hypertension, systolic blood pressure, diabetes mellitus, cholesterol, and fasting glucose. In separate models we further adjusted for comedication use or level of the aminoterminal fragment of pro-B-type natriuretic peptide (NT-proBNP) and cardiac troponin T.
Two restricted cohorts were considered in fully-adjusted analyses to further address the possibility of confounding. To account for confounding by indication (in which the underlying disease of gastroesophageal reflux, rather than treatment, could be linked to incident disease or LV morphology), analyses were repeated in a restricted cohort comparing participants who used H2RAs to those who used proton pump inhibitors (8). In a second cohort, to account for confounding related to a participant's likelihood to use H2RAs, propensity scores were used to match H2RA users with nonusers of a similar propensity to use H2RAs. The propensity to use H2RAs was calculated as a logit function including factors hypothesized to predict H2RA use, such as comorbidity, body mass index (BMI), and insurance status (see the Online Appendix for all covariate choices and matching scheme).
Exploratory analyses evaluated associations between H2RA use and heart failure in groups with differing predicted HF risk. These analyses were predicated on the hypothesis that, if a treatment effect exists, it might be most apparent in individuals at the highest risk for heart failure. A HF risk score derived from the ARIC (Atherosclerosis Risk in Communities) study was estimated for each participant with available measures using baseline characteristics (17). The percent of participants with incident HF at any point during follow-up for a given predicted baseline risk (determined by the ARIC risk score) was evaluated using locally-weighted scatterplot smoothing (LOWESS). This allowed a depiction of the observed HF incidence in groups of individuals with different predicted risk at baseline. Kaplan-Meier cumulative event curves, HF incidence, relative risk, and risk difference were also presented in stratified cohorts with high (ARIC risk score >11) and low (ARIC risk score ≤11) risk at baseline. Further exploratory models evaluated whether age, sex, BMI category, beta-blocker use, or baseline NT-proBNP level modified associations between H2RA use and heart failure.
In the primary analyses, H2RA exposure was evaluated at the earliest available time point (baseline) since the etiologic window of H2RA use relevant to HF is unknown, but may be protracted given potential biologic mechanisms involving cardiac fibrosis or remodeling. To test the validity of this assumption for H2RA use and explore timing and duration of H2RA use, time-varying exposure models characterized the association between active/current H2RA use with incident HF (Online Figure 1 and Online Appendix).
Linear regression was used to characterize associations between H2RA use at the baseline exam, initial LV parameters, and change in LV parameters over time (e.g., baseline LV mass subtracted from LV mass at the follow-up CMR). Models with limited adjustment, full adjustment, and full adjustment with comedication use were considered in both full and restricted cohorts. We adjusted for the sum of LV parameters (e.g., baseline LV mass added to LV mass at the follow-up CMR) in all models of LV change to address concerns that change in LV parameters was confounded by baseline differences. This allowed inference on LV change independent of differences in the absolute magnitude of LV measures. We used consistency of H2RA use (nonuse, use during any single MESA examination, or use noted during at least 4 MESA examinations) as a plausible surrogate for cumulative dose over time in analyses of LV change and presented these results as marginal predictions from fully-adjusted linear regression models. Analyses were performed using STATA 12.0 (StataCorp, College Station, Texas).
Results
Of 6,814 participants enrolled in MESA, follow-up for incident HF was obtained for 6,783 individuals. Covariates were missing in 405 participants, leaving 6,378 subjects in the cohort evaluated for incident HF (94% of all participants). Of these, 4,691 completed a baseline CMR and were included in the cohort describing cross-sectional associations between H2RA use and the LV. A follow-up CMR was obtained in 2,806 participants (107 H2RA users and 2,699 nonusers) a median of 9.4 years after the first CMR (interquartile range [IQR]: 9.1 to 9.7 years). These participants were included in the cohort describing associations between H2RA use and longitudinal changes in the LV (Figure 1).
Figure 1. Study Sample.
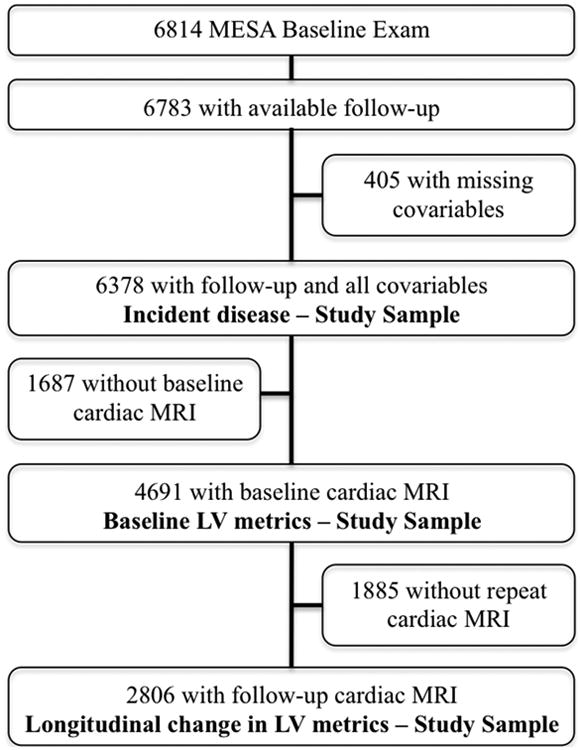
Flow diagram characterizes MESA participants who contributed to each of the analysis cohorts evaluating the relationships between H2 receptor antagonists (H2RA) and heart failure (HF), baseline cardiac morphology, and change in cardiac morphology over time. MESA = Multi-Ethnic Study of Atherosclerosis.
The mean age of the study sample was 62.3 years, 52.7% were women, and 37.9% were white. The 313 H2RA users in the cohort evaluated for incident HF tended to be older and heavier, were more likely to be white and to use other non-H2RA medications, and had a higher prevalence of hypertension than 6,065 nonusers (Table 1). Of 313 H2RA users, 170 (54.3%) reported use at only 1 MESA exam and 47 (15.0%) reported H2RA use at 4 or more MESA exams. Median follow-up was 11.2 years (IQR: 10.6 to 11.7 years) and total follow-up was 65,082 person-years. In unadjusted analyses, 6 H2RA users developed HF in 3,082 person-years (1.9 events per 1,000 person-years) and 230 nonusers developed HF in 62,000 person-years (3.7 events per 1,000 person-years) (p value from a log-rank test of the Kaplan-Meier survival function = 0.11) (Central Illustration).
Table 1. Baseline Characteristics.
| H2RA Users (n = 313) | H2RA Nonusers (n = 6,065) | p Value | |
|---|---|---|---|
| Age, yrs | 65.2 ± 9.9 | 62.1 ± 10.2 | <0.001 |
| Female | 52.1 | 52.7 | 0.84 |
| Race | |||
| White | 43.8 | 37.6 | |
| Chinese | 8.0 | 12.5 | 0.008 |
| African-American | 23.3 | 28.0 | |
| Hispanic | 24.9 | 21.9 | |
| Height, cm | 165.8 ± 9.8 | 166.3 ± 10.0 | 0.44 |
| Weight, kg | 80.3 ± 16.7 | 78.5 ± 17.4 | 0.06 |
| Body mass index, kg/m2 | 29.2 ± 5.6 | 28.3 ± 5.5 | 0.005 |
| Educational attainment | |||
| No high school degree | 18.5 | 18.2 | |
| High school degree | 22.4 | 17.8 | |
| Some college | 17.6 | 16.2 | 0.26 |
| Bachelor's degree | 17.6 | 17.5 | |
| Higher than bachelor's degree | 13.4 | 18.2 | |
| Cigarette smoking status | |||
| Never | 48.9 | 51.0 | |
| Former | 39.0 | 36.2 | 0.62 |
| Current | 12.1 | 12.8 | |
| Pack-years | 16.3 ± 26.6 | 11.0 ± 20.4 | <0.001 |
| Hypertension | 54.0 | 44.8 | 0.002 |
| Systolic blood pressure, mm Hg | 129.3 ± 20.4 | 126.5 ± 21.6 | 0.03 |
| Diabetes mellitus | 14.4 | 13.6 | 0.71 |
| Cholesterol, mg/dl | 192 (169-214) | 192 (170-215) | 0.46 |
| Glucose, mg/dl | 91 (84-101) | 90 (83-99) | 0.11 |
| NT-proBNP, pg/ml* | 66 (30-121) | 54 (24-112) | 0.02 |
| Medications | |||
| NSAIDs | 54.3 | 40.1 | <0.001 |
| Oral steroids | 3.8 | 1.4 | 0.003 |
| Beta-blockers | 12.8 | 9.4 | 0.001 |
| ACE inhibitors/ARBs | 25.6 | 18.0 | 0.001 |
| Any diuretic | 19.2 | 13.3 | 0.005 |
Values are mean ± SD, percentage, or median (interquartile range); differences were evaluated with Student t test, Fisher exact test, or Wilcoxon rank sum test respectively.
Available for 5,285 participants.
ACE = angiotensin-converting enzyme; ARB = angiotensin II receptor blocker; H2RA = H2 receptor antagonist; NSAIDs = nonsteroidal anti-inflammatory drugs; NT-proBNP = N-terminal pro–B-type natriuretic peptide.
Central Illustration. H2-receptor antagonists and heart failure: Unadjusted associations between H2 receptor antagonists (H2RA) use and heart failure.
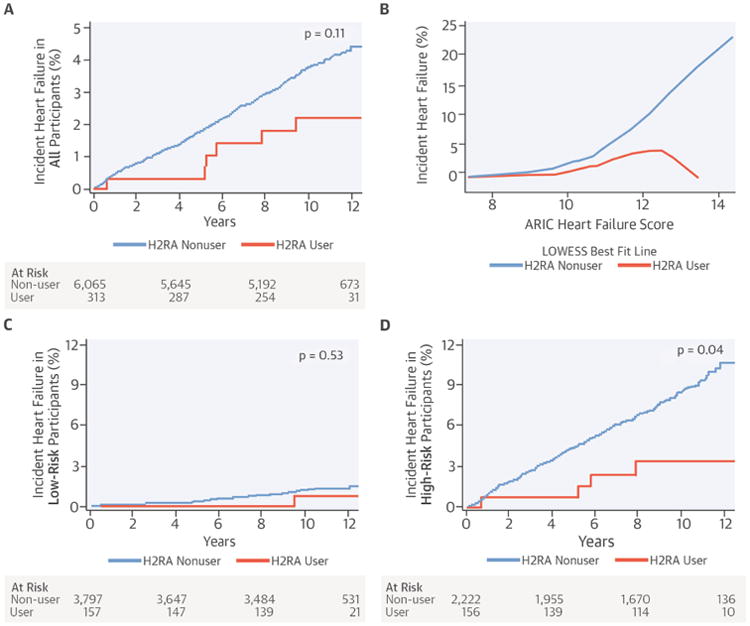
(A) unadjusted Kaplan-Meier cumulative event curve for incident heart failure in all participants, (B) locally weighted scatterplot smoothing (LOWESS) comparing incident heart failure occurring at any point during follow-up relative to the baseline predicted risk of heart failure (predicted by the ARIC heart failure score), (C) unadjusted Kaplan-Meier cumulative event curve for incident heart failure in participants at LOW risk for heart failure (predicted by an ARIC heart failure score ≤ 11), (D) and unadjusted Kaplan-Meier cumulative event curve for incident heart failure in participants at HIGH risk for heart failure (predicted by an ARIC heart failure score >11)
ARIC = Atherosclerosis Risk in Communities.
H2RA use at the baseline exam was associated with a 62% lower risk of HF (adjusted hazard ratio [HR]: 0.38; 95% confidence interval [CI]: 0.17 to 0.86; p = 0.02) (Table 2). This association was stronger when accounting for NT-proBNP and troponin T at baseline (adjusted HR: 0.18; 95% CI: 0.05 to 0.62; p = 0.007( (Table 2). Associations were similar with adjustment for comedication use, when the cohort was restricted to propensity-matched participants, and when the cohort was restricted to participants who used either H2RAs or proton pump inhibitors (Table 2).
Table 2. Association of H2RA Use at Baseline and HF.
| Adjusted Risk of HF in H2RA Users Relative to Nonusers (N = 6,378) | |||
|---|---|---|---|
| Hazard Ratio | 95% CI | p Value | |
| Unadjusted | 0.52 | 0.23 to 1.18 | 0.12 |
| Limited adjustment* | 0.40 | 0.18 to 0.90 | 0.03 |
| Full adjustment† | 0.38 | 0.17 to 0.86 | 0.02 |
| Full adjustment† + comedication use‡ | 0.35 | 0.16 to 0.80 | 0.01 |
| Full adjustment† + NT-proBNP & troponin T (n = 5,285) | 0.18 | 0.05 to 0.62 | 0.007 |
| Restricted to H2RA users and PPI users§ (n = 698) | 0.42 | 0.15 to 1.16 | 0.09 |
| Restricted to PS matched participants§ (n = 593) | 0.31 | 0.11 to 0.86 | 0.03 |
Limited adjustment accounts for age, sex, race/ethnicity, height, weight, and study site.
Full adjustment accounts for the limited model and education, cigarette smoking, pack-years, hypertension, systolic blood pressure, diabetes, cholesterol, glucose, and daily exercise.
Comedication use included NSAIDs (aspirin, Cox-2 inhibitors, and other nonsteroidal inflammatory medications), steroids, beta-blockers (± diuretics), ACE inhibitors (± diuretics), ARBs (± diuretics), any diuretic alone (including potassium-sparking diuretics), leukotriene antagonists, and digoxin.
Participants in the restricted cohorts were considered in models with full adjustment.
CI = confidence interval; PPI = proton pump inhibitor; PS = propensity score; other abbreviations as in Table 1.
The largest unadjusted risk difference for heart failure was seen in participants with the highest predicted risk for HF at baseline (Central Illustration). H2RA users with low predicted HF risk (ARIC score ≤11) had a relative risk for heart failure of 0.5 and experienced 0.5 fewer episodes of incident HF per 1,000 person-years than nonusers. H2RA users with higher HF risk (ARIC score >11) had a relative risk for heart failure of 0.4 and experienced 5.3 fewer episodes of incident HF per 1,000 person-years (Central Illustration).
Age, sex, BMI and beta-blocker use did not modify associations between H2RA use and HF (p for the interaction: 0.37, 0.13, 0.49, and 0.25 respectively). Similar to results stratified by HF risk score, baseline NT-proBNP was an effect modifier of the association between H2RA use and HF (p < 0.001). Individuals with a higher level of NT-proBNP at baseline had a greater reduction in the hazard of heart failure with H2RA use relative to individuals with a lower level of NT-proBNP at baseline. Use of time-varying exposure models to describe associations between active H2RA use and clinical outcomes suggested a more modest association between concurrent “active” H2RA use and HF development (HR: 0.75; 95% CI: 0.37 to 1.51; p = 0.42). The association was stronger but still not statistically significant when H2RA use was restricted to participants with at least 1 year of H2RA use (HR: 0.51: 95% CI: 0.21 to 1.23; p = 0.13) (Online Tables 1 and 2 for adjusted and unadjusted associations incorporating time varying definitions of H2RA exposure).
H2RA use was associated with differences in baseline CMR. After adjustment for covariates, H2RA use was associated with a smaller LV end-diastolic volume (LVEDV) (-5.7 ml; 95% CI: -8.8 to -2.6 ml, p < 0.001), smaller stroke volume (-3.5 ml; 95% CI: -5.6 to -1.4 ml; p = 0.001), and a higher mass/volume ratio (0.04 g/ml; 95% CI: 0.02 to 0.07 g/ml; p < 0.001). Associations were unchanged with adjustment for comedication use and in the propensity-matched cohort. Restriction to participants who used H2RAs or proton pump inhibitors attenuated associations between H2RA use and baseline CMR metrics (Online Table 3). There was no association between H2RA use and baseline LV ejection fraction or mass.
H2RA use was associated with blunted LV change over time. Among participants who completed both CMRs, LVEDV decreased, LV stroke volume decreased, and mass/volume ratio increased over time. Compared to nonusers, H2RA users had a smaller decline in LVEDV (4.6 ml; 95% CI: 0.4 to 8.8 ml; p = 0.03), a smaller decline in LV stroke volume (3.9 ml; 95% CI: 0.7 to 7.1 ml; p = 0.02) and a smaller increase in mass/volume ratio (-0.05 g/ml; 95% CI: -0.09 to -0.02 g/ml; p = 0.005) over time (Figure 2). Results were not different after further adjustment for comedication use and in the propensity-matched cohort. Restricting analyses to participants with gastroesophageal reflux disease attenuated associations with LV change (Online Table 4). Increasing consistency of H2RA use over time suggested progressively stronger associations with change in LVEDV, LV stroke volume, and mass/volume ratio (Table 3).
Figure 2. Change in Cardiac Morphology over time in H2RA users relative to non-users.
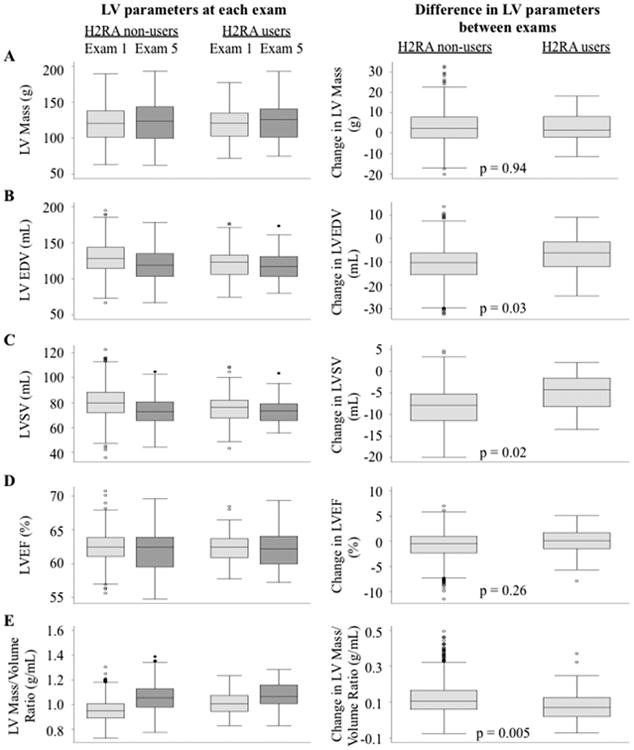
Change in left ventricular (LV) (A) mass, (B) end-diastolic volume (EDV), (C) stroke volume (SV), (D) ejection fraction (EF), and (E) mass/volume ratio over approximately 10 years can be seen among users and nonusers of H2RAs. Other abbreviations as in Figure 1.
Table 3. Consistency of H2RA Use and LV Changes*.
| Change in LV Parameters Relative to Baseline Exam (n = 2,806) | ||
|---|---|---|
| Change | 95% CI | |
| Change in LV end-diastolic volume | ||
| No H2RA use | -10.9 ml | -11.7 to -10.0 mL |
| H2RA use at only 1 study exam | -8.8 ml | -14.4 to -3.2 mL |
| Consistent H2RA use at 4+ study exams | 0.8 ml | -8.1 to 9.6 mL |
| Test of trend: | p = 0.02 | |
| Change in LV ejection fraction | ||
| No H2RA use | -0.7% | -1.0 to -0.4 |
| H2RA use at only 1ne study exam | -0.1% | -1.9 to 1.8 |
| Consistent H2RA use at 4+ study exams | 0.0% | -3.0 to 2.9 |
| Test of trend: | p = 0.61 | |
| Change in LV stroke volume | ||
| No H2RA use | -8.3 ml | -9.0 to -7.7 |
| H2RA use at only 1 study exam | -5.8 ml | -10.1 to -1.5 |
| Consistent H2RA use at 4+ study exams | -0.8 ml | -7.6 to 5.9 |
| Test of trend: | p = 0.04 | |
| Change in LV mass | ||
| No H2RA use | 2.9 g | 2.2 to 3.6 g |
| H2RA use at only 1 study exam | 0.7 g | -3.8 to 5.2 g |
| Consistent H2RA use at 4+ study exams | 6.3 g | -0.8 to 13.3 |
| Test of trend: | p = 0.60 | |
| Change in LV Mass/Volume Ratio | ||
| No H2RA use | 0.12 g/ml | 0.11 to 0.13 |
| H2RA use at only one study exam | 0.08 g/ml | 0.03 to 0.13 |
| Consistent H2RA use at 4+ study exams | 0.04 g/ml | -0.03 to 0.12 |
| Test of trend: | p = 0.02 | |
Changes between the initial and the follow-up cardiac magnetic resonance imaging (CMR) exam.
All models fully adjusted for age, sex, race/ethnicity, height, weight, study site, the sum of the left ventricular (LV) parameter of interest at CMR 1 and CMR 2, education, cigarette smoking, pack-years, hypertension, systolic blood pressure, diabetes, cholesterol, glucose, and daily exercise.
Discussion
In the current study, H2RA use was associated with a lower risk for incident heart failure in a multiethnic cohort without CVD at baseline. The strongest association (in absolute terms) was seen in individuals at highest risk for HF. H2RA users had smaller LVEDV and stroke volume at baseline, but over time, H2RA use was associated with a smaller decline in stroke volume, a smaller decline in LVEDV, and a smaller increase in the LV mass/volume ratio.
The association between baseline H2RA use and incident HF persisted regardless of the method used to account for confounding. The cohort matched by propensity to use H2RAs suggested H2RA use, and not characteristics of H2RA users, was responsible for the association. Comparing H2RA users to proton pump inhibitor users again suggested H2RA use, and not the presence of gastroesophageal reflux disease, was responsible for the association. Multivariate models argue that measured differences in cardiovascular risk factors between groups do not explain the reduced HF risk. In fact, there was a particularly large association between H2RA use and HF when adjusting for NT-proBNP (one of the strongest predictors of incident heart failure) and in participants with a high risk of HF as predicted by their ARIC HF risk score (17,18). These findings joined previous observations in animal models to suggest a biologically plausible histamine-related mechanism important to the pathogenesis of heart failure (4,6,7).
This is the first report to suggest H2RA use is associated with decreased HF incidence in humans, but not the first to suggest H2RA use may be important for the heart. Among 318 Japanese participants with HFrEF, use of famotidine (an H2RA) was associated with better New York Heart Association functional class, lower BNP, and a smaller LVEDV (9). In a subsequent prospective randomized trial of 50 participants with HF, Kim et al. compared famotidine to teprenone use over 24 weeks and found improved functional class, decreased BNP, and decreased LV diameter in the famotidine arm (9).
Improved HF symptoms may be related, but are distinct from our description of decreased HF incidence, and our study suggested important differences relative to these previous studies. Among participants with HFrEF, randomized famotidine use was associated with a smaller LV diameter. This agrees with our current cross-sectional observation of a smaller LVEDV among H2RA users, but differs from our finding of a smaller decline in LVEDV over time among H2RA users. This discrepancy may reflect the difference in duration (10 years vs. 24 weeks) and differences in the population being studied. The famotidine trial studied participants with HFrEF where a decrease in the size of a pathologically enlarged LV was a marker of improvement (19). MESA participants were initially free of clinical CVD and without a pathologically enlarged LV at baseline. Some developed HFrEF, some developed HF with preserved ejection fraction (HFpEF), but most did not develop heart failure. Decrease in LV size is not always a marker of improvement. In HFpEF, decreased LV size is associated with disease progression, hospitalization, and death (20). Protection from an age-related decrease in LV size appears better aligned with the current findings and protection from HFpEF might provide a rationale for our observations. Pathophysiology relevant to HFpEF may be further suggested by the less pronounced increase in mass/volume ratio over time in H2RA users, which may imply less concentric remodeling, and the lack of an association between H2RA use and ejection fraction (21,22). Currently there are no beneficial therapies for HFpEF, which is common and serious (23). Although highly speculative based on the current findings, further study of histamine signaling in the pathogenesis of HFpEF may be warranted.
Timing and duration of H2RA use are likely important. Associations with LV morphology were strongest in individuals who reported consistent H2RA use over several MESA exams. Also, while baseline H2RA use was strongly related to HF risk, “active use” was only weakly related to incident HF unless “active use” was restricted to participants who used H2RAs for at least 1 year, possibly suggesting that benefits with early, prolonged, and/or consistent H2RA use exist that are not present with short-term use or use beginning during later stages of HF pathogenesis.
Study Limitations
Associations with cardiac morphology were less consistent than those with heart failure, particularly after restricting to participants with gastroesophageal reflux disease. Furthermore, H2RA users had smaller LVEDV and LV stroke volume at baseline but less decline in these measures over time, which could represent regression to the mean. Regression to the mean would not explain associations with HF and small sample size likely contributed to inconsistency after restriction to participants with gastroesophageal reflux disease; however, inference on cardiac structure and a paradigm of “protection” from age-related changes in cardiac morphology, which informed our discussion of mechanism, should be more cautious. In addition, HF is a heterogeneous disease. Because of the small number of HF events among H2RA users, this study could not reliably characterize important differences between types of HF relative to H2RA use. Importantly this included an inability to characterize differences in HFpEF relative to HFrEF. Timing and duration of H2RA use may be important, but could not be fully evaluated as MESA did not assess medication use more frequently than every other year and medication use before the baseline exam was not known. Residual confounding, unmeasured confounding, and misclassification in a nonrandomized study are always possible and for this reason inference on pharmaco-epidemiology must be cautious. The relatively small sample size of participants who used H2RAs might lead to less stable estimates of HF incidence than would be seen in a larger population, which further reinforces the need for cautious inference when interpreting the current findings.
Conclusions
H2RA use was associated with a lower risk for incident HF and better preserved stroke volume, LVEDV, and mass/volume ratio over time in community-dwelling adults. Histamine signaling may be important in the pathogenesis of HF and could be an important target for therapy or prevention of heart failure.
Supplementary Material
Perspectives.
Competency in Medical Knowledge
Histamine signaling may be important in the pathogenesis of heart failure by inhibiting myocardial fibrosis and apoptosis, and in a cohort observed over 10 years the use of H2 receptor antagonists was associated with a lower the risk of incident heart failure.
Translational Outlook
Randomized trials are needed to verify whether the use of H2 receptor antagonists can influence the development or progression of heart failure and clarify the mechanisms responsible for this effect.
Acknowledgments
MESA Investigators reviewed the manuscript for scientific content and consistency of data interpretation with previous MESA publications. Significant comments were incorporated before submission for publication. We thank investigators, staff, and participants of MESA for their valuable contributions. A full list of participating MESA Investigators and institutions can be found at http://www.mesa-nhlbi.org.
Funding/Support: This research was supported by contracts N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute and by grants UL1-TR-000040 and UL1-TR-001079 from NCRR. This publication was supported by the National Center For Advancing Translational Sciences of the National Institutes of Health under Award KL2TR000421.
Abbreviations
- CMR
cardiac magnetic resonance imaging
- EDV
end-diastolic volume
- H2RA
histamine H2 receptor antagonists
- HFpEF
heart failure with preserved ejection fraction
- HFrEF
heart failure with reduced ejection fraction
- LV
left ventricle
- NT-proBNP
N-terminal pro-B-type natriuretic peptide
Footnotes
Clinical trials identifier: NCT00005487
Disclosures: No author reported a conflict of interest related to this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jacobson BC, Ferris TG, Shea TL, et al. Who is using chronic acid suppression therapy and why? Am J Gastroenterol. 2003;98:51–8. doi: 10.1111/j.1572-0241.2003.07186.x. [DOI] [PubMed] [Google Scholar]
- 2.Bristow MR, Ginsburg R, Harrison DC. Histamine and the human heart: the other receptor system. Am J Cardiol. 1982;49:249–51. doi: 10.1016/0002-9149(82)90298-3. [DOI] [PubMed] [Google Scholar]
- 3.Matsuda N, Jesmin S, Takahashi Y, et al. Histamine H1 and H2 receptor gene and protein levels are differentially expressed in the hearts of rodents and humans. J Pharmacol Exp Ther. 2004;309:786–95. doi: 10.1124/jpet.103.063065. [DOI] [PubMed] [Google Scholar]
- 4.Bristow MR, Minobe WA, Billingham ME, et al. Anthracycline-associated cardiac and renal damage in rabbits. Evidence for mediation by vasoactive substances. Lab Invest. 1981;45:157–68. [PubMed] [Google Scholar]
- 5.Bristow MR, Kantrowitz NE, Harrison WD, Minobe WA, Sageman WS, Billingham ME. Mediation of subacute anthracycline cardiotoxicity in rabbits by cardiac histamine release. J Cardiovasc Pharmacol. 1983;5:913–9. doi: 10.1097/00005344-198311000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Takahama H, Asanuma H, Sanada S, et al. A histamine H2 receptor blocker ameliorates development of heart failure in dogs independently of β-adrenergic receptor blockade. Basic Res Cardiol. 2010;105:787–94. doi: 10.1007/s00395-010-0119-y. [DOI] [PubMed] [Google Scholar]
- 7.Zeng Z, Shen L, Li X, et al. Disruption of histamine H2 receptor slows heart failure progression through reducing myocardial apoptosis and fibrosis. Clin Sci. 2014;127:435–48. doi: 10.1042/CS20130716. [DOI] [PubMed] [Google Scholar]
- 8.Luo T, Chen B, Zhao Z, et al. Histamine H2 receptor activation exacerbates myocardial ischemia/reperfusion injury by disturbing mitochondrial and endothelial function. Basic Res Cardiol. 2013;108:342. doi: 10.1007/s00395-013-0342-4. [DOI] [PubMed] [Google Scholar]
- 9.Kim J, Ogai A, Nakatani S, et al. Impact of blockade of histamine H2 receptors on chronic heart failure revealed by retrospective and prospective randomized studies. J Am Coll Cardiol. 2006;48:1378–84. doi: 10.1016/j.jacc.2006.05.069. [DOI] [PubMed] [Google Scholar]
- 10.Leary PJ, Barr RG, Bluemke DA, et al. H2 receptor antagonists and right ventricular morphology: The MESA Right Ventricle Study. Ann Am Thorac Soc. 2014;11:1379–86. doi: 10.1513/AnnalsATS.201407-344OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bild DE, Bluemke DA, Burke GL, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–81. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 12.Venkatesh BA, Volpe GJ, Donekal S, et al. Association of longitudinal changes in left ventricular structure and function with myocardial fibrosis: the Multi-Ethnic Study of Atherosclerosis study. Hypertension. 2014;64:508–15. doi: 10.1161/HYPERTENSIONAHA.114.03697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Natori S, Lai S, Finn JP, et al. Cardiovascular function in multi-ethnic study of atherosclerosis: normal values by age, sex, and ethnicity. Am J Roentgenol. 2006;186:S357–65. doi: 10.2214/AJR.04.1868. [DOI] [PubMed] [Google Scholar]
- 14.Bluemke DA, Kronmal RA, Lima JA, et al. The relationship of left ventricular mass and geometry to incident cardiovascular events: the MESA (Multi-Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol. 2008;52:2148–55. doi: 10.1016/j.jacc.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith NL, Psaty BM, Heckbert SR, Tracy RP, Cornell ES. The reliability of medication inventory methods compared to serum levels of cardiovascular drugs in the elderly. J Clin Epidemiol. 1999;52:143–6. doi: 10.1016/s0895-4356(98)00141-3. [DOI] [PubMed] [Google Scholar]
- 16.MESA Manual of Operations: Field Center and Laboratory Procedures. [Accessed March 17, 2014]; No authors listed. Available from: http://www.mesa-nhlbi.org/manuals.aspx.
- 17.Agarwal SK, Chambless LE, Ballantyne CM, et al. Prediction of Incident Heart Failure in General Practice: The Atherosclerosis Risk in Communities (ARIC) Study. Circ Heart Fail. 2012;5:422–9. doi: 10.1161/CIRCHEARTFAILURE.111.964841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nambi V, Liu X, Chambless LE, et al. Troponin T and N-Terminal Pro-B-Type Natriuretic Peptide: A Biomarker Approach to Predict Heart Failure Risk--The Atherosclerosis Risk in Communities Study. Clin Chem. 2013;59:1802–10. doi: 10.1373/clinchem.2013.203638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kramer DG, Trikalinos TA, Kent DM, Antonopoulos GV, Konstam MA, Udelson JE. Quantitative evaluation of drug or device effects on ventricular remodeling as predictors of therapeutic effects on mortality in patients with heart failure and reduced ejection fraction: a meta-analytic approach. J Am Coll Cardiol. 2010;56:392–406. doi: 10.1016/j.jacc.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burke MA, Katz DH, Beussink L, et al. Prognostic importance of pathophysiologic markers in patients with heart failure and preserved ejection fraction. Circ Heart Fail. 2014;7:288–99. doi: 10.1161/CIRCHEARTFAILURE.113.000854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zile MR, Gottdiener JS, Hetzel SJ, et al. Prevalence and significance of alterations in cardiac structure and function in patients with heart failure and a preserved ejection fraction. Circulation. 2011;124:2491–501. doi: 10.1161/CIRCULATIONAHA.110.011031. [DOI] [PubMed] [Google Scholar]
- 22.Gaasch WH, Zile MR. Left ventricular structural remodeling in health and disease: with special emphasis on volume, mass, and geometry. J Am Coll Cardiol. 2011;58:1733–40. doi: 10.1016/j.jacc.2011.07.022. [DOI] [PubMed] [Google Scholar]
- 23.Raina A, Kanwar M. New Drugs and Devices in the Pipeline for Heart Failure with Reduced Ejection Fraction Versus Heart Failure with Preserved Ejection Fraction. Curr Heart Fail Rep. 2014;11:374–81. doi: 10.1007/s11897-014-0222-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


