Abstract
Background: Expression of PD-L1 has been estimated to predict the therapeutic potential of PD-L1 inhibition in solid tumors. Recent studies have demonstrated that PD-L1 plays a critical role in regulatory T-cell (Treg) development and functional maintenance. Although increases in FOXP3+Treg infiltration and PD-L1 expression have been revealed in several malignancies, their correlation in human breast tumors is as yet unclear.
Methods: Whole-tissue sections from 501 patients with breast cancer were examined for PD-L1 and FOXP3 expression by immunohistochemistry. Correlation between their expressions and the association with clinicopathological features, intrinsic tumor subtypes and patient's prognosis were studied.
Results: PD-L1 expression and FOXP3+Treg infiltrates in tumor tissue demonstrated a high correlation (rs=0.334, p<0.001) in this cohort of breast cancer patients. High PD-L1 expression and increased FOXP3+Treg infiltrates were both associated with high histological grade, negative ER and PR status, and aggressive intrinsic tumor subtypes, especially the basal-like subtype. Tumors with concomitant high expressions of the two markers had the worst prognosis. Multivariate analysis proved both markers to be the independent predictors for decreased overall survival of patients, particularly in the basal-like subtype.
Conclusions: The results suggest that PD-L1 and FOXP3+Tregs may work synergistically and their up-regulated expressions promote tumor immune evasion in breast cancer. Combinatorial immunotherapeutic approaches aiming on blocking PD-L1 and depleting Tregs might improve therapeutic efficacy in breast cancer patients, especially those with basal-like carcinoma.
Keywords: Breast cancer, FOXP3+Tregs, PD-L1, Intrinsic subtype, Prognosis.
Introduction
Programmed death ligand 1 (PD-L1) is a member of the B7 superfamily. It is a 40-kDa transmembrane protein that is encoded by CD274 gene located on chromosome 9 1. Upregulation of PD-L1 has been described in several malignancies and closely associated with the clinicopathological status of patients with solid tumors 2-4. Previous studies have demonstrated abundant PD-L1 molecules were expressed by both tumor cells and infiltrating immune cells and its inhibition result in an enduring clinical response in recent clinical trials of several solid tumors 5-8. Recently, PD-L1 expression has been studied to predict clinical response to PD-L1 inhibition in order to estimate the patients who may benefit from the therapy 6. However, its expression and impact on the prognosis of patients with breast cancer is controversial in the limited reports 9-12.
Regulatory T cells (Tregs), a unique subset of CD4+ helper T cells characterized by the CD4+ CD25+ phenotype, can suppress proliferation and cytokine secretion of effector T lymphocytes through immunoregulation. FOXP3, a forkhead helix transcription factor, appears to function as a master regulator in the development and control of Tregs 13, 14, and is regarded as the most specific and reliable surface marker of Tregs 15-17. FOXP3 is considered a biomarker and prognostic factor for human malignant tumors 18. In our previous study with 1,270 samples of whole-tissue sections, intratumoral infiltration by FOXP3+ Tregs was highly correlated with the intrinsic subtype and was revealed as an independent prognostic predictor for breast cancer patients 19, 20.
Persuasive evidence has suggested that PD-L1 plays a pivotal role in the induction and maintenance of Tregs that leads to expansion of Tregs in tumor microenvironment and these induced Tregs (iTregs) then inhibit T cell responses to tumor 21-24. In vitro, PD-L1-coated beads can induce Tregs in the absence of exogenous TGF-β, indicating that PD-L1 signaling can facilitate the development of iTregs 21. In vivo, blocking PD-L1 signaling abolished induction in a tumor-induced Treg conversion model 25. So far, except for these in vitro or animal models, the link between PD-L1 expression in tumor cell and the infiltration of Tregs has been evaluated in patients with gastric and colorectal carcinoma 26-28. However, this relevancy is as yet unclear in patients with breast cancer.
In present study, we evaluated the expression of PD-L1 and the infiltration of FOXP3+ Treg in whole-tissue sections from a large cohort of 501 breast carcinomas, and further investigated their association with the clinicopathological features of the tumors, the intrinsic tumor subtypes and the prognosis of patients, according to REMARK recommendations 29.
Materials and methods
Specimen selection and clinical information
We selected 501 continuous cases of invasive breast carcinoma diagnosed at the Department of Breast Cancer Pathology, Tianjin Medical University Cancer Institute and Hospital, Tianjin, China in 2004 that were included in the prior study 19. It included 439 invasive ductal carcinomas not otherwise specified (NOS-IDC), 18 invasive lobular carcinomas (ILC) and 44 carcinomas of other histologic types. The patients were followed up for 1-80 months, with a median of 64 months, and the median age of the patients at diagnosis was 53 years (range 29-83 years). All the patients presented with tumors that were confined to the breast, without evidence of distant metastasis or skin involvement, and all underwent surgical excision with axillary lymph node dissection 19. No patients had received neoadjuvant chemotherapy or preoperative radiation therapy. Postoperatively, 461 (92.0%) patients received adjuvant chemotherapy, 195 (38.9%) received radiation therapy, and 350 (69.9%) received endocrine therapy. Patient's consent for research was obtained prior to surgery and the research was given official approval by the Institutional Research and Ethical Committee of Tianjin Medical University Cancer Institute and Hospital.
Immunohistochemistry
Immunohistochemistry for PD-L1 and FOXP3 was performed on whole-tissue sections using standard procedures. Briefly, 4-µm tissue sections were sequentially dewaxed and rehydrated using xylene and graded alcohol washes. Antigen retrieval was performed at 121°C for 2 min using citrate buffer, pH 6.0. After serial blocking with hydrogen peroxide and normal goat serum, the sections were incubated with a primary polyclonal antibody against PD-L1 (Abcam, ab58810, polyclonal, 1:500 dilution, Cambridge, UK) for 16 h at 4°C. The sections were then sequentially incubated with biotinylated goat anti-mouse immunoglobulin and peroxidase-conjugated streptavidin (DAKO). The enzyme substrate was 3,3'-diaminobenzidine tetra-hydrochloride. Incubation of sections with phosphate-buffered saline alone served as a negative control.
PD-L1 expression in cytoplasm and/or on cellular membrane of tumor cells was considered positive and was quantified using the Histo-score (H-score) system by evaluation of the entire slide. A case was scored by the intensity (1+ = weak, 2+ = moderate, 3+ = strong) (Fig. 1) and the percentage of staining in invasive tumor cells. The H-score was calculated using the following formula: (3 × percentage of cells strong staining) + (2 × percentage of cell with moderate staining) + (1 × percentage of cells with weak staining), with the possible scores ranging from 0 to 300. PD-L1 expression was classified into two groups according to a cut-off H-score of 100 (0-99 = negative expression; 100-300 = positive expression).
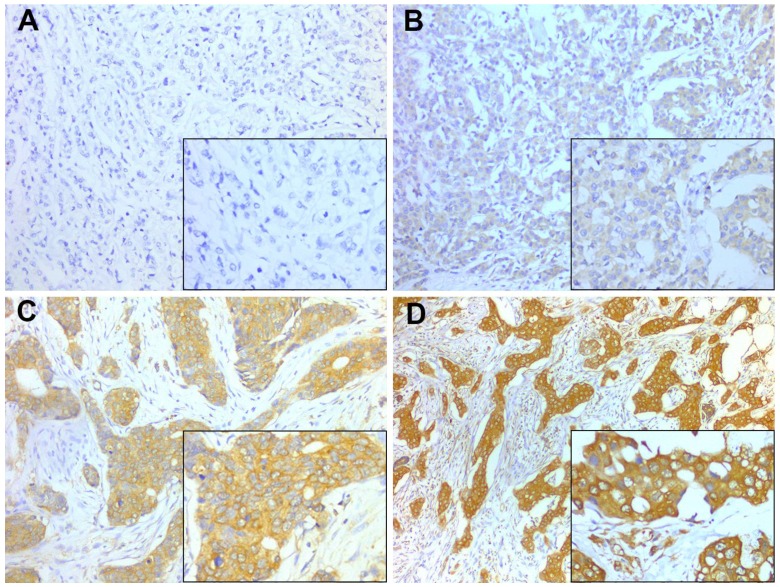
Figure 1.
Representative PD-L1 staining intensities. Staining was localized to the cytoplasm and to the membrane of breast cancer cells. A, Intensity 0, no staining; B, Intensity 1+, weak staining; C, Intensity 2+, moderate staining; D, Intensity 3+, strong staining. A-D, original magnification × 200; inset ×400.
Immunohistochemistry for FOXP3 and its scoring were described in prior study 19. As previously described, the infiltrating density of intratumoral FOXP3+ Tregs was categorized as high or low relative to the median of 11 cells/0.0625 mm2 as the cutoff value.
Statistical analysis
Statistical analyses were performed with SPSS 18.0 software (SPSS, Chicago, IL). Spearman's rank-correlation test was used to assess the association of PD-L1 expression with FOXP3+ Treg infiltration and clinicopathological characteristics. Chi-square tests were used to compare PD-L1 expression and Treg infiltration among intrinsic subtypes. The cumulative survival (overall survival, OS; recurrence-free survival, RFS) times were calculated using the Kaplan-Meier method and analyzed with the log-rank test. Univariate and multivariate analyses were conducted based on the Cox proportional hazards regression model. All tests were two-sided, and a P value of less than 0.05 was considered statistically significant.
Results
Correlation of PD-L1 expression with FOXP3+ Treg infiltration and clinicopathological features
Among the 501 invasive breast cancer patient samples, 231 cases (46.1%) exhibited PD-L1 expression and 271 (54.1%) cases exhibited high FOXP3+ Treg infiltration. Significant correlation between PD-L1 expression and FOXP3+ Treg infiltration in breast cancer tissue was identified (rs=0.334, p<0.001) (Table 1).
Table 1.
Associations between PD-L1 expression and clinicopathological parameters.
| PD-L1 expression | FOXP3+ Tregs | |||||||
|---|---|---|---|---|---|---|---|---|
| Clinicopathological parameters |
Negative n (%) |
Positive n (%) |
rs | P* |
Low n(%) |
High n(%) |
rs | P* |
|
No. of patients Age, years |
270(53.9) | 231(46.1) | 230(45.9) | 271(54.1) | ||||
| < 50 | 142(57.7) | 104(42.3) | 0.075 | 0.091 | 116(47.2) | 130(52.8) | 0.025 | 0.583 |
| ≥50 | 128(50.2) | 127(49.8) | 114(44.7) | 141(55.3) | ||||
| Tumor size, cm | ||||||||
| ≤2 | 76(55.1) | 62(44.9) | 0.015 | 0.744 | 68(49.3) | 70(50.7) | 0.042 | 0.352 |
| > 2 | 194(53.4) | 169(46.6) | 162(44.6) | 201(55.4) | ||||
| Histological grade | ||||||||
| Grade 1 | 22(57.9) | 16(42.1) | 0.118 | 0.008 | 29(76.3) | 9(23.7) | 0.208 | <0.001 |
| Grade 2 | 192(57.7) | 141(42.3) | 159(47.7) | 174(52.3) | ||||
| Grade 3 | 56(43.1) | 74(56.9) | 42(32.3) | 88(67.7) | ||||
| Lymph node status | ||||||||
| Negative | 136(58.4) | 97(41.6) | 0.125 | 0.005 | 110(47.2) | 123(52.8) | 0.057 | 0.203 |
| 1 to 3 | 67(59.8) | 45(40.2) | 57(50.9) | 55(49.1) | ||||
| 4 to 9 | 33(45.8) | 39(54.2) | 35(48.6) | 37(51.4) | ||||
| 10 or more | 34(40.5) | 50(59.5) | 29(34.5) | 55(65.5) | ||||
| ER status | ||||||||
| Negative | 76(45.2) | 92(54.8) | -0.123 | 0.006 | 33(19.6) | 135(80.4) | -0.374 | <0.001 |
| Positive | 194(58.3) | 139(41.7) | 197(59.2) | 136(40.8) | ||||
| PR status | ||||||||
| Negative | 93(44.7) | 115(55.3) | -0.155 | <0.001 | 53(25.5) | 155(74.5) | -0.345 | <0.001 |
| Positive | 177(60.4) | 116(39.6) | 177(60.4) | 116(39.6) | ||||
| HER-2 status | ||||||||
| Negative | 218(55.5) | 175(44.5) | 1.828 | 0.176 | 192(48.9) | 201(51.1) | 0.113 | 0.012 |
| Positive | 52(48.1) | 56(51.9) | 38(35.2) | 70(64.8) | ||||
| Molecular subtypes | ||||||||
| Luminal A | 116(63.0) | 68(37.0) | 0.008** | 125(67.9) | 59(32.1) | <0.001** | ||
| Luminal B | 64(54.2) | 54(45.8) | 60(50.8) | 58(49.2) | ||||
| Luminal HER-2 | 35(51.5) | 33(48.5) | 25(36.8) | 43(63.2) | ||||
| HER-2-enriched | 17(42.5) | 23(57.5) | 10(25.0) | 30(75.0) | ||||
| Basal-like | 38(41.8) | 53(58.2) | 10(11.0) | 81(89.0) | ||||
|
FOXP3+ Tregs (cells / 0.0625 mm2) |
||||||||
| Low (<11) | 171(71.2) | 69(28.8) | 0.334 | <0.001 | ||||
| High (≥11) | 99(37.9) | 162(62.1) | ||||||
*P-values were calculated by Spearman's rank-correlation test to assess the association of PD-L1 expression and FOXP3+ Treg infiltration with clinicopathological characteristics.
**PD-L1 expression and FOXP3+ Treg infiltration were compared between intrinsic subtypes by the Chi-square test (χ2=13.821; χ2=90.941).
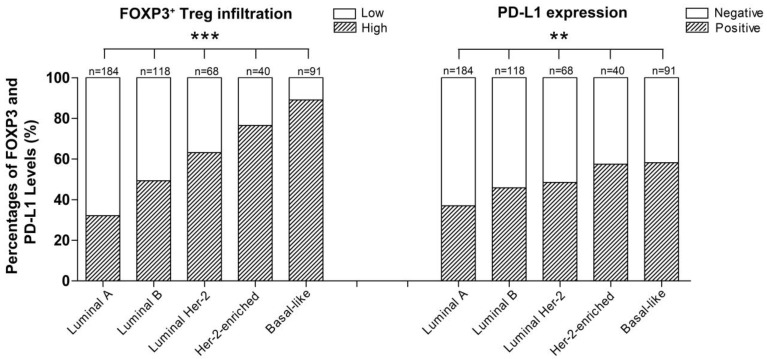
Moreover, PD-L1 expression and FOXP3+ Treg infiltration were both positively associated with a high histological grade (rs=0.118, p=0.008; rs=0.208, p<0.001), negative ER (rs=-0.123, p=0.006; rs=-0.374, p<0.001) and PR status (rs=-0.155, p<0.001; rs=-0.345, p<0.001), and the intrinsic subtype of breast cancer (χ2=13.821, p=0.008; χ2=90.941, p=0.001) (Table 1). Interestingly, the order of intrinsic subtypes in which the rate of FOXP3+ Treg infiltration increased (Luminal A < Luminal B < Luminal HER2 < HER2-enriched < Basal-like breast cancer) was entirely consistent with the order in which the PD-L1 expression rate increased (Table 1, Fig. 2). The expression of the two proteins were both inversely correlated with the intrinsic subtypes of carcinomas in term of their known prognostic significance.
Figure 2.
Percentage of patients with different breast cancer intrinsic subtypes exhibiting different levels of FOXP3+ Treg infiltration (***p<0.001) and PD-L1 expression (**p=0.008).
Prognostic significance of PD-L1 expression and FOXP3+ Treg infiltration
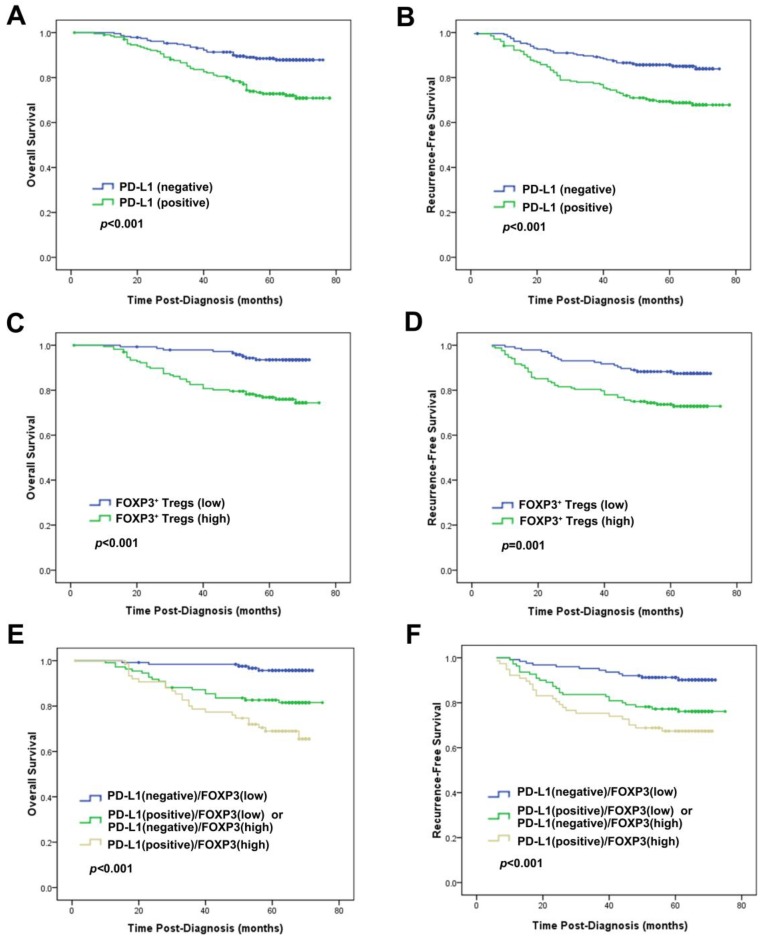
Univariate analysis revealed that PD-L1 expression was an unfavorable predictor for OS and RFS (HR=2.544, p<0.001; HR=2.262, p<0.001) (Table 2; Fig. 3). Consistent with the results in prior study 19, infiltration of FOXP3+ Tregs was also an unfavorable predictor of OS and RFS in univariate analysis (HR=4.330, P<0.001; HR=2.418, P=0.002) (Table 2; Fig. 3). At multivariate analysis, after adjusting for age, tumor size, grade, lymph node stage, ER, PR and HER2 status, chemotherapy, radiotherapy and endocrine therapy, both PD-L1 expression and FOXP3+ Treg infiltration were independent prognostic factors for OS (HR=1.874, p=0.044; HR=3.178, p=0.003, respectively) and RFS (HR=1.725, p=0.044; HR=2.114, p=0.009, respectively) (Table 3).
Table 2.
Univariate analysis of pathological features, PD-L1 expression and FOXP3+ Treginfiltration with OS and RFS in breast cancer patients
| OS | RFS | |||||
|---|---|---|---|---|---|---|
| Variable | HR | 95% CI | P | HR | 95% CI | P |
| Age, years (<50 vs.≥50) | 1.017 | 0.661-1.564 | 0.939 | 0.984 | 0.664-1.460 | 0.937 |
| Tumor size, cm (≤2 vs. >2) | 1.188 | 0.718-1.967 | 0.502 | 1.076 | 0.682-1.695 | 0.754 |
| Histological Grade (I vs. II vs. III) | 2.116 | 1.413-3.171 | <0.001 | 2.075 | 1.437-2.997 | <0.001 |
| Lymph node stage(N0 vs. N1 vs. N2 vs. N3) | 2.103 | 1.725-2.563 | <0.001 | 2.045 | 1.709-2.446 | <0.001 |
| ER status (negative vs.positive) | 0.389 | 0.251-0.600 | <0.001 | 0.482 | 0.325-0.716 | <0.001 |
| PR status s(negative vs. positive) | 0.437 | 0.279-0.684 | <0.001 | 0.542 | 0.363-0.808 | 0.003 |
| HER-2 status (negative vs. positive) | 2.087 | 1.315-3.314 | <0.001 | 1.753 | 1.135-2.709 | 0.011 |
| PD-L1 (negative vs. positive) | 2.544 | 1.607-4.028 | <0.001 | 2.262 | 1.498-3.416 | <0.001 |
| FOXP3+ Tregs (low vs. high) | 4.330 | 2.101-8.926 | <0.001 | 2.418 | 1.400-4.178 | 0.002 |
| Chemotherapy (No vs. Yes) | 6.393 | 0.890-45.932 | 0.065 | 7.929 | 1.106-56.859 | 0.039 |
| Radiotherapy (No vs. Yes) | 2.596 | 1.474-4.572 | 0.001 | 2.649 | 1.608-4.364 | <0.001 |
| Endocrine therapy (No vs. Yes) | 0.358 | 0.233-0.552 | <0.001 | 0.470 | 0.314-0.703 | <0.001 |
Lymph node stage: N0, indicates no lymph node metastasis; N1, 1-3 lymph node metastasis; N2, 4-9 lymph node metastasis; N3, ≥10 lymph node metastasis.
HR, hazard ratio; CI, confidence interval; OS: overall survival; RFS: recurrence-free survival.
Figure 3.
Prognostic significance of PD-L1 expression alone or combined with evaluation of FOXP3+ Tregs in breast cancer patients. Kaplan-Meier survival curve for (A) OS and (B) RFS depending on the expression of PD-L1. (C) OS and (D) RFS depending on the tumor FOXP3+ Treg infiltration. (E) OS and (F) RFS depending on the PD-L1 expression combined with the tumor FOXP3+ Treg infiltration. p-values were calculated by the log-rank test.
Table 3.
Multivariate analysis of pathological features, PD-L1 expression and FOXP3+ Treg infiltration with OS and RFS in breast cancer patients.
| OS | RFS | ||||||
|---|---|---|---|---|---|---|---|
| Variable | HR | 95% CI | P | HR | 95% CI | P | |
| Age, years (<50 vs.≥50) | 0.924 | 0.600-1.424 | 0.720 | 0.902 | 0.607-1.341 | 0.611 | |
| Tumor size, cm (≤2 vs. >2) | 1.237 | 0.747-2.048 | 0.409 | 1.107 | 0.682-1.796 | 0.681 | |
| Histological Grade (I vs. II vs. III) | 1.546 | 1.017-2.350 | 0.042 | 1.562 | 1.072-2.276 | 0.020 | |
| Lymph node stage (N0 vs. N1 vs. N2 vs. N3) | 2.030 | 1.650-2.498 | <0.001 | 1.994 | 1.653-2.405 | <0.001 | |
| ER status (negative vs.positive) | 0.559 | 0.332-0.940 | 0.028 | 0.584 | 0.362-0.941 | 0.027 | |
| PR statu s(negative vs.positive) | 0.888 | 0.376-2.095 | 0.785 | 0.892 | 0.444-1.796 | 0.750 | |
| HER-2 status (negative vs. positive) | 1.843 | 1.141-2.979 | 0.012 | 1.940 | 1.127-3.340 | 0.017 | |
| PD-L1 (negative vs. positive) | 1.874 | 1.018-3.451 | 0.044 | 1.725 | 1.015-2.929 | 0.044 | |
| FOXP3+ Tregs (low vs. high) | 3.178 | 1.477-6.893 | 0.003 | 2.114 | 1.205-3.706 | 0.009 | |
| Chemotherapy (No vs. Yes) | 1.067 | 0.134-8.529 | 0.951 | 1.946 | 0.252-15.01 | 0.523 | |
| Radiotherapy (No vs. Yes) | 0.466 | 0.192-1.131 | 0.091 | 0.486 | 0.215-1.101 | 0.084 | |
| Endocrine therapy (No vs. Yes) | 0.532 | 0.131-2.156 | 0.377 | 0.607 | 0.180-2.050 | 0.421 | |
Lymph node stage: N0, indicates no lymph node metastasis; N1, 1-3 lymph node metastasis; N2, 4-9 lymph node metastasis; N3, ≥10 lymph node metastasis.
HR, hazard ratio; CI, confidence interval; OS: overall survival; RFS: recurrence-free survival.
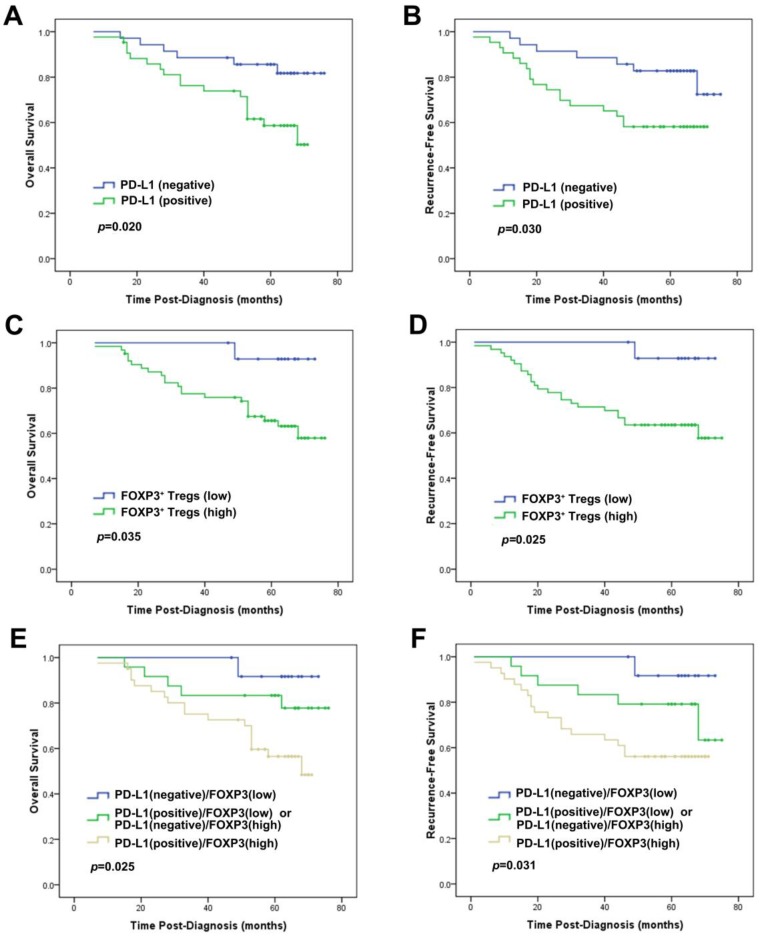
In subset analyses by intrinsic subtypes, PD-L1 expression was associated with decreased OS and/or RFS in the luminal A, luminal B and basal-like subtypes by univariate and multivariate analysis (Table 4). FOXP3+ Treg infiltration was associated with decreased OS and/or RFS in the luminal-HER2, HER2-enriched and basal-like subtypes by univariate and multivariate analysis (Table 5). We noticed that, in the basal-like subtype, both PD-L1 (HR=2.600, p=0.046) and FOXP3+ Tregs (HR=8.139, p=0.043) proved to be independent unfavorable prognostic factors for OS by multivariate analysis adjusting by age, tumor size, grade and lymph node status (Table 4 and 5, Fig. 4).
Table 4.
Univariate and multivariate analyses of intrinsic subtypes regarding the effect of PD-L1 expression on OS and RFS in breast cancer patients.
| OS | RFS | |||||
|---|---|---|---|---|---|---|
| Intrinsic subtype | HR | 95% CI | P | HR | 95% CI | P |
| Univariate analysis | ||||||
| Luminal A | 4.287 | 1.072-17.145 | 0.040 | 2.346 | 0.953-5.774 | 0.064 |
| Luminal B | 3.300 | 1.051-10.366 | 0.041 | 3.998 | 1.030-12.265 | 0.015 |
| Luminal HER2 | 3.016 | 0.928-9.807 | 0.066 | 2.068 | 0.735-5.817 | 0.168 |
| HER2-enriched | 3.601 | 0.798-16.255 | 0.096 | 4.353 | 0.982-19.298 | 0.053 |
| Basal-like | 2.835 | 1.124-7.153 | 0.027 | 2.537 | 1.058-6.082 | 0.037 |
| Multivariate analysis* | ||||||
| Luminal A | 4.118 | 1.029-16.472 | 0.045 | 2.437 | 0.982-6.051 | 0.055 |
| Luminal B | 3.078 | 0.970-9.772 | 0.056 | 3.471 | 1.112-10.831 | 0.032 |
| Luminal HER2 | 2.990 | 0.898-9.961 | 0.074 | 2.054 | 0.713-5.912 | 0.182 |
| HER2-enriched | 3.558 | 0.773-16.371 | 0.103 | 4.243 | 0.946-19.033 | 0.059 |
| Basal-like | 2.600 | 1.016-6.652 | 0.046 | 2.317 | 0.956-5.615 | 0.063 |
*The multivariate analysis of PD-L1 expression in each intrinsic subtype was adjusted by age, tumor size, histological grade, lymph node status and FOXP3+ Tregs
HR, hazard ratio; CI, confidence interval; OS: overall survival; RFS: recurrence-free survival.
Table 5.
Univariate and multivariate analyses of intrinsic subtypes regarding the effect of FOXP3+ Tregs on OS and RFS in breast cancer patients
| OS | RFS | ||||||
|---|---|---|---|---|---|---|---|
| Intrinsic subtype | HR | 95% CI | P | HR | 95% CI | P | |
| Univariate analysis | |||||||
| Luminal A | 2.000 | 0.745-5.371 | 0.169 | 1.883 | 0.881-4.023 | 0.102 | |
| Luminal B | 3.769 | 0.800-17.753 | 0.093 | 2.309 | 0.813-6.557 | 0.116 | |
| Luminal HER2 | 4.704 | 1.272-17.395 | 0.020 | 3.998 | 1.250-12.785 | 0.019 | |
| HER2-enriched | 3.763 | 1.193-11.869 | 0.024 | 2.559 | 0.895-7.317 | 0.080 | |
| Basal-like | 7.740 | 1.044-57.355 | 0.045 | 8.387 | 1.134-62.036 | 0.037 | |
| Multivariate analysis* | |||||||
| Luminal A | 1.988 | 0.740-5.340 | 0.173 | 1.906 | 0.892-4.072 | 0.096 | |
| Luminal B | 3.339 | 0.709-15.724 | 0.127 | 2.130 | 0.750-6.050 | 0.156 | |
| Luminal HER2 | 6.076 | 1.525-24.208 | 0.011 | 5.641 | 1.640-19.402 | 0.006 | |
| HER2-enriched | 4.475 | 1.148-17.452 | 0.031 | 2.756 | 0.958-7.932 | 0.060 | |
| Basal-like | 8.139 | 1.070-61.895 | 0.043 | 6.482 | 0.838-50.142 | 0.073 | |
*The multivariate analysis of FOXP3+ Tregs in each intrinsic subtype was adjusted by age, tumor size, histological grade, lymph node status and PD-L1 expression
HR, hazard ratio; CI, confidence interval; OS: overall survival; RFS: recurrence-free survival.
Figure 4.
Prognostic significance of PD-L1 expression alone or combined with FOXP3+ Treg infiltration in patients with the basal-like subtype of breast cancer. Kaplan-Meier survival curves for (A) OS and (B) RFS depending on the expression of PD-L1. (C) OS and (D) RFS depending on the tumor FOXP3+ Treg infiltration. (E) OS and (F) RFS depending on the PD-L1 expression combined with the tumor FOXP3+ Treg infiltration. p-values were calculated by the log-rank test.
Prognostic significance of concomitant PD-L1 expression and FOXP3+ Tregs
One hundred and sixty-two (32.3%) tumors showed concurrence of PD-L1 expression and increased FOXP3+ Treg infiltration, while 171 (34.1%) tumors exhibited negative PD-L1 expression and decreased FOXP3+ Treg infiltration, and the other 168 (33.5%) tumors demonstrated neither of the above (Table 1).
The group of the patients with the concomitant PD-L1 expression and increased FOXP3+ Treg infiltration showed the worst OS and RFS, while those with negative PD-L1 and low FOXP3+ Tregs demonstrated the best OS and RFS among the 3 groups. Patients with other combinative pattern of PD-L1 expression and Tregs infiltration exhibited OS and RFS in the middle of the groups (OS: χ2=27.937, p<0.001; RFS: χ2=17.467, p<0.001; Fig. 3). This result was particularly proved to be significant in the basal-like subtype (OS: χ2=7.387, p=0.025; RFS: χ2=6.950, p=0.031; Fig. 4).
Discussion
It has become clear that malignant cells must be able to successfully avoid immune surveillance to progress and metastasize. PD-L1, a co-inhibitory molecule, seems a major contributor to this process. PD-L1 induces cancer cell immune evasion by binding to its receptor PD-1 on activated T cells that results in tolerance of tumor-reactive T cells 30, 31, and in rendering tumor cells to be resistant to CD8+ T cells. FASL-mediated tumor cell lysis is also inhibited by PD1 and PD-L1 interaction 32. Recently, PD-L1 has been considered as an unfavorable predictor in a variety of malignancies 9, 11, 33-39.
Two prior studies using tissue microarrays (TMAs) of large sample size obtained quite different PD-L1 protein expression rates and opposite associations with clinical outcome of breast cancer 11, 40. Ail et al reported PD-L1 expressed by tumor cell occurred in just 1.7% of tumors and associated with improved disease-specific survival. In contrast, Muenst et al. 11 found PD-L1 expression in 23.4 % of specimens and reported its association with higher tumor grade, lymph node metastasis, diminished ER expression, and decreased OS. Considering the limitations of TMAs, including their inability to represent PD-L1 expression and to capture the immune infiltration accurately due to intra-tumoral heterogeneity, we chose to use the whole-tissue sections for the evaluation of PD-L1 expression in 501 breast cancer samples and an H-score system was adopted for accurate scoring. The whole-tissue sections allowed reliable observation of tumor FOXP3+ Tregs infiltration and PD-L1 expression that facilitate the concomitant evaluation of the two markers per our previous experiences 19. In this study we found PD-L1 expressed by tumor cell in 46.1% of specimens which was even higher than 23.4% reported by Muenst et al. 11 used the same antibody clone and scoring system except for using TMA samples. However, their results of associations with vital clinicopathological parameters and patient prognosis were consistent with our findings. In addition, we also demonstrated a significant correlation between PD-L1 expression and the intrinsic molecular subtypes of the carcinomas. In two studies employing 44 and 69 cases of breast cancer separately 9, 10, Ghebeh et al. also identified that PD-L1 expression in tumor cells were associated with a higher tumor grade and negative ER and PR status. However, in their studies, no significant correlation with lymph node metastasis was found, and no follow-up information was provided.
Two prior studies evaluated PD-L1 at the level of gene expression 12, 41. Schalper et al. 12 reported that 60% of breast tumors showed CD274 (PD-L1) mRNA expression in TMAs and was associated with increased tumor-infiltrating lymphocytes (TILs) and improved recurrence-free survival of breast cancer patients. Sabatier et al. 41 report that CD274 (PD-L1) was upregulated in 20% of tumors based on DNA microarray data and was not associated with survival in the whole population. However, the limitations of the sampling effect of TMAs and the possibility of poor correlation between PD-L1 RNA and protein expression should be considered. The authors also suggested that the translation of these findings into the clinical setting could benefit from whole-tissue-section estimation or in combination with PD-L1 protein detection.
Profound evidence has shown that Tregs and the PD-L1/PD1 pathway are pivotal to the maintenance of peripheral immune tolerance, and may involve in the same pathway. A novel role of PD-L1 in sustaining the function of iTregs has been suggested. PD-L1 was found to modulate the signaling molecules that are critical for the conversion of naive T cells to Treg cells 42, 43. It can upregulate FOXP3 expression and therefore intensify its suppressive function 44. One previous report documented that the coexistence of FOXP3+ TILs and PD-L1+ TILs in breast cancer tissues is significantly correlated with unfavorable patient prognostic factors 45. The pivotal finding of the present study is to reveal the congregation of tumor cell PD-L1 expression and FOXP3+ Treg infiltration in the tumor microenvironment of breast cancer. The results pointed out to a possibility that PD-L1 signals might play an important role in immune suppression through regulating Tregs in the complicated suppressive network of breast cancer, although the accurate mechanism deserves is still elusive.
Ongoing clinical trials targeting the potential PD-L1/PD-1 pathway have shown that PD-L1 inhibitors are safe, well-tolerated and have few autoimmune side effects 5, 46, 47. The development of new strategy is particularly urgent in the management of basal-like carcinoma patients where therapeutic options are still remarkably limited. Multiple previous observations, both in cell lines and in tumor samples are concordant with our finding of enriched PD-L1 in basal-like breast cancer 11, 33, 34, 40, 41. Recently, several clinical trials targeting the PD-L1/PD-1 axis in basal-like carcinoma have achieved tumor pathologically complete responses and improved patient's outcomes 48-50. In present study, we found that both PD-L1 and FOXP3 showed their highest levels and strong associations with poor prognosis in the basal-like subtype. The combined evaluation identified that patients with the concomitant presence of high PD-L1 expression and high FOXP3+ Treg infiltration had the worst prognosis. Given the limited options for basal-like subtype treatment, a combined strategy to block PD-L1/PD-1 axis with simultaneous depletion of Tregs should be reasonable in enhancing the therapeutic efficacy of these patients. The potential benefits of this model have been demonstrated in a recent study of mouse renal cell carcinoma, in which mice showed long-lasting protective immunity and complete tumor regression when such a therapeutic modality was applied 51. Despite these promising results, the specific implication of PD-L1 inhibitors on Treg function has not been reported and should be further explored in future studies of breast cancer.
Conclusion
The study demonstrates a significant correlation between two independent poor prognostic indicators of breast cancer: the tumor cell high PD-L1 expression and the increased tissue FOXP3+ Treg infiltrates. The results also suggest that PD-L1 and FOXP3+ Tregs work synergistically or participate in the same molecular pathway and their up-regulated expressions promote tumor immune evasion. The findings provide a theoretical basis for the development of immunotherapies targeting PD-L1 and FOXP3+ Tregs simultaneously in the treatment of breast cancer, especially the basal-like subtype carcinoma. These immunosuppressive molecules should be further explored to facilitate the development of anti-tumor immunotherapies for breast cancer.
Acknowledgments
Research supported by grants from the National Natural Science Foundation of China (Grant No. 81202101, 81302292, 81302294 and 31400673) and Natural Science Foundation of Tianjin City (Grant No. 15JCQNJC45300).
Abbreviations
- PD-L1
programmed death ligand 1
- Treg
regulatory T-cell
- TILs
tumor-infiltrating lymphocytes
- ER
estrogen receptor
- PR
progesterone receptor
- HER-2
human epidermal growth factor receptor 2
- RFS
recurrence-free survival
- OS
overall survival
- HR
hazard ratio
- CI
confidence interval.
References
- 1.Butte MJ, Pena-Cruz V, Kim MJ, Freeman GJ, Sharpe AH. Interaction of human PD-L1 and B7-1. Molecular immunology. 2008;45:3567–72. doi: 10.1016/j.molimm.2008.05.014. doi:10.1016/j.molimm.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Droeser RA, Hirt C, Viehl CT, Frey DM, Nebiker C, Huber X. et al. Clinical impact of programmed cell death ligand 1 expression in colorectal cancer. European journal of cancer. 2013;49:2233–42. doi: 10.1016/j.ejca.2013.02.015. doi:10.1016/j.ejca.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 3.Chen YB, Mu CY, Huang JA. Clinical significance of programmed death-1 ligand-1 expression in patients with non-small cell lung cancer: a 5-year-follow-up study. Tumori. 2012;98:751–5. doi: 10.1177/030089161209800612. doi:10.1700/1217.13499. [DOI] [PubMed] [Google Scholar]
- 4.Hamanishi J, Mandai M, Iwasaki M, Okazaki T, Tanaka Y, Yamaguchi K. et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:3360–5. doi: 10.1073/pnas.0611533104. doi:10.1073/pnas.0611533104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P. et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. The New England journal of medicine. 2012;366:2455–65. doi: 10.1056/NEJMoa1200694. doi:10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS. et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–7. doi: 10.1038/nature14011. doi:10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB. et al. Improved survival with ipilimumab in patients with metastatic melanoma. The New England journal of medicine. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. doi:10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Powles T, Eder JP, Fine GD, Braiteh FS, Loriot Y, Cruz C. et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515:558–62. doi: 10.1038/nature13904. doi:10.1038/nature13904. [DOI] [PubMed] [Google Scholar]
- 9.Ghebeh H, Mohammed S, Al-Omair A, Qattan A, Lehe C, Al-Qudaihi G. et al. The B7-H1 (PD-L1) T lymphocyte-inhibitory molecule is expressed in breast cancer patients with infiltrating ductal carcinoma: correlation with important high-risk prognostic factors. Neoplasia. 2006;8:190–8. doi: 10.1593/neo.05733. doi:10.1593/neo.05733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghebeh H, Tulbah A, Mohammed S, Elkum N, Bin Amer SM, Al-Tweigeri T. et al. Expression of B7-H1 in breast cancer patients is strongly associated with high proliferative Ki-67-expressing tumor cells. International journal of cancer Journal international du cancer. 2007;121:751–8. doi: 10.1002/ijc.22703. doi:10.1002/ijc.22703. [DOI] [PubMed] [Google Scholar]
- 11.Muenst S, Schaerli AR, Gao F, Daster S, Trella E, Droeser RA. et al. Expression of programmed death ligand 1 (PD-L1) is associated with poor prognosis in human breast cancer. Breast cancer research and treatment. 2014;146:15–24. doi: 10.1007/s10549-014-2988-5. doi:10.1007/s10549-014-2988-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schalper KA, Velcheti V, Carvajal D, Wimberly H, Brown J, Pusztai L. et al. In situ tumor PD-L1 mRNA expression is associated with increased TILs and better outcome in breast carcinomas. Clinical cancer research: an official journal of the American Association for Cancer Research. 2014;20:2773–82. doi: 10.1158/1078-0432.CCR-13-2702. doi:10.1158/1078-0432.CCR-13-2702. [DOI] [PubMed] [Google Scholar]
- 13.Yagi H, Nomura T, Nakamura K, Yamazaki S, Kitawaki T, Hori S. et al. Crucial role of FOXP3 in the development and function of human CD25+CD4+ regulatory T cells. International immunology. 2004;16:1643–56. doi: 10.1093/intimm/dxh165. doi:10.1093/intimm/dxh165. [DOI] [PubMed] [Google Scholar]
- 14.Campbell DJ, Ziegler SF. FOXP3 modifies the phenotypic and functional properties of regulatory T cells. Nature reviews Immunology. 2007;7:305–10. doi: 10.1038/nri2061. doi:10.1038/nri2061. [DOI] [PubMed] [Google Scholar]
- 15.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nature immunology. 2003;4:330–6. doi: 10.1038/ni904. doi:10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 16.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–61. doi: 10.1126/science.1079490. doi:10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 17.Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nature immunology. 2003;4:337–42. doi: 10.1038/ni909. doi:10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 18.Schreiber TH. The use of FoxP3 as a biomarker and prognostic factor for malignant human tumors. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2007;16:1931–4. doi: 10.1158/1055-9965.EPI-07-0396. doi:10.1158/1055-9965.EPI-07-0396. [DOI] [PubMed] [Google Scholar]
- 19.Liu F, Lang R, Zhao J, Zhang X, Pringle GA, Fan Y. et al. CD8(+) cytotoxic T cell and FOXP3(+) regulatory T cell infiltration in relation to breast cancer survival and molecular subtypes. Breast cancer research and treatment. 2011;130:645–55. doi: 10.1007/s10549-011-1647-3. doi:10.1007/s10549-011-1647-3. [DOI] [PubMed] [Google Scholar]
- 20.Liu F, Li Y, Ren M, Zhang X, Guo X, Lang R. et al. Peritumoral FOXP3(+) regulatory T cell is sensitive to chemotherapy while intratumoral FOXP3(+) regulatory T cell is prognostic predictor of breast cancer patients. Breast cancer research and treatment. 2012;135:459–67. doi: 10.1007/s10549-012-2132-3. doi:10.1007/s10549-012-2132-3. [DOI] [PubMed] [Google Scholar]
- 21.Francisco LM, Salinas VH, Brown KE, Vanguri VK, Freeman GJ, Kuchroo VK. et al. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206:3015–29. doi: 10.1084/jem.20090847. doi:10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krupnick AS, Gelman AE, Barchet W, Richardson S, Kreisel FH, Turka LA. et al. Murine vascular endothelium activates and induces the generation of allogeneic CD4+25+Foxp3+ regulatory T cells. Journal of immunology. 2005;175:6265–70. doi: 10.4049/jimmunol.175.10.6265. [DOI] [PubMed] [Google Scholar]
- 23.Park JJ, Omiya R, Matsumura Y, Sakoda Y, Kuramasu A, Augustine MM. et al. B7-H1/CD80 interaction is required for the induction and maintenance of peripheral T-cell tolerance. Blood. 2010;116:1291–8. doi: 10.1182/blood-2010-01-265975. doi:10.1182/blood-2010-01-265975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ni XY, Sui HX, Liu Y, Ke SZ, Wang YN, Gao FG. TGF-beta of lung cancer microenvironment upregulates B7H1 and GITRL expression in dendritic cells and is associated with regulatory T cell generation. Oncology reports. 2012;28:615–21. doi: 10.3892/or.2012.1822. doi:10.3892/or.2012.1822. [DOI] [PubMed] [Google Scholar]
- 25.Wang L, Pino-Lagos K, de Vries VC, Guleria I, Sayegh MH, Noelle RJ. Programmed death 1 ligand signaling regulates the generation of adaptive Foxp3+CD4+ regulatory T cells. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:9331–6. doi: 10.1073/pnas.0710441105. doi:10.1073/pnas.0710441105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao LW, Li C, Zhang RL, Xue HG, Zhang FX, Zhang F. et al. B7-H1 and B7-H4 expression in colorectal carcinoma: correlation with tumor FOXP3(+) regulatory T-cell infiltration. Acta histochemica. 2014;116:1163–8. doi: 10.1016/j.acthis.2014.06.003. doi:10.1016/j.acthis.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 27.Hou J, Yu Z, Xiang R, Li C, Wang L, Chen S. et al. Correlation between infiltration of FOXP3+ regulatory T cells and expression of B7-H1 in the tumor tissues of gastric cancer. Experimental and molecular pathology. 2014;96:284–91. doi: 10.1016/j.yexmp.2014.03.005. doi:10.1016/j.yexmp.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 28.Geng Y, Wang H, Lu C, Li Q, Xu B, Jiang J. et al. Expression of costimulatory molecules B7-H1, B7-H4 and Foxp3(+) Tregs in gastric cancer and its clinical significance. International journal of clinical oncology. 2015;20:273–81. doi: 10.1007/s10147-014-0701-7. doi:10.1007/s10147-014-0701-7. [DOI] [PubMed] [Google Scholar]
- 29.McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM. et al. REporting recommendations for tumor MARKer prognostic studies (REMARK) Breast cancer research and treatment. 2006;100:229–35. doi: 10.1007/s10549-006-9242-8. doi:10.1007/s10549-006-9242-8. [DOI] [PubMed] [Google Scholar]
- 30.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB. et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nature medicine. 2002;8:793–800. doi: 10.1038/nm730. doi:10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 31.Latchman YE, Liang SC, Wu Y, Chernova T, Sobel RA, Klemm M. et al. PD-L1-deficient mice show that PD-L1 on T cells, antigen-presenting cells, and host tissues negatively regulates T cells. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:10691–6. doi: 10.1073/pnas.0307252101. doi:10.1073/pnas.0307252101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Azuma T, Yao S, Zhu G, Flies AS, Flies SJ, Chen L. B7-H1 is a ubiquitous antiapoptotic receptor on cancer cells. Blood. 2008;111:3635–43. doi: 10.1182/blood-2007-11-123141. doi:10.1182/blood-2007-11-123141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soliman H, Khalil F, Antonia S. PD-L1 expression is increased in a subset of basal type breast cancer cells. PloS one. 2014;9:e88557.. doi: 10.1371/journal.pone.0088557. doi:10.1371/journal.pone.0088557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mittendorf EA, Philips AV, Meric-Bernstam F, Qiao N, Wu Y, Harrington S. et al. PD-L1 expression in triple-negative breast cancer. Cancer immunology research. 2014;2:361–70. doi: 10.1158/2326-6066.CIR-13-0127. doi:10.1158/2326-6066.CIR-13-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zang X, Allison JP. The B7 family and cancer therapy: costimulation and coinhibition. Clinical cancer research: an official journal of the American Association for Cancer Research. 2007;13:5271–9. doi: 10.1158/1078-0432.CCR-07-1030. doi:10.1158/1078-0432.CCR-07-1030. [DOI] [PubMed] [Google Scholar]
- 36.Hino R, Kabashima K, Kato Y, Yagi H, Nakamura M, Honjo T. et al. Tumor cell expression of programmed cell death-1 ligand 1 is a prognostic factor for malignant melanoma. Cancer. 2010;116:1757–66. doi: 10.1002/cncr.24899. doi:10.1002/cncr.24899. [DOI] [PubMed] [Google Scholar]
- 37.Hasan A, Ghebeh H, Lehe C, Ahmad R, Dermime S. Therapeutic targeting of B7-H1 in breast cancer. Expert opinion on therapeutic targets. 2011;15:1211–25. doi: 10.1517/14728222.2011.613826. doi:10.1517/14728222.2011.613826. [DOI] [PubMed] [Google Scholar]
- 38.Mu CY, Huang JA, Chen Y, Chen C, Zhang XG. High expression of PD-L1 in lung cancer may contribute to poor prognosis and tumor cells immune escape through suppressing tumor infiltrating dendritic cells maturation. Medical oncology. 2011;28:682–8. doi: 10.1007/s12032-010-9515-2. doi:10.1007/s12032-010-9515-2. [DOI] [PubMed] [Google Scholar]
- 39.Song M, Chen D, Lu B, Wang C, Zhang J, Huang L. et al. PTEN loss increases PD-L1 protein expression and affects the correlation between PD-L1 expression and clinical parameters in colorectal cancer. PloS one. 2013;8:e65821.. doi: 10.1371/journal.pone.0065821. doi:10.1371/journal.pone.0065821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ali HR, Glont SE, Blows FM, Provenzano E, Dawson SJ, Liu B. et al. PD-L1 protein expression in breast cancer is rare, enriched in basal-like tumours and associated with infiltrating lymphocytes. Annals of oncology: official journal of the European Society for Medical Oncology / ESMO. 2015 doi: 10.1093/annonc/mdv192. doi:10.1093/annonc/mdv192. [DOI] [PubMed] [Google Scholar]
- 41.Sabatier R, Finetti P, Mamessier E, Adelaide J, Chaffanet M, Ali HR. et al. Prognostic and predictive value of PDL1 expression in breast cancer. Oncotarget. 2015;6:5449–64. doi: 10.18632/oncotarget.3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burton OT, Zaccone P, Phillips JM, De La Pena H, Fehervari Z, Azuma M. et al. Roles for TGF-beta and programmed cell death 1 ligand 1 in regulatory T cell expansion and diabetes suppression by zymosan in nonobese diabetic mice. Journal of immunology. 2010;185:2754–62. doi: 10.4049/jimmunol.1001365. doi:10.4049/jimmunol.1001365. [DOI] [PubMed] [Google Scholar]
- 43.Zhao Y, Huang Z, Qi M, Lazzarini P, Mazzone T. Immune regulation of T lymphocyte by a newly characterized human umbilical cord blood stem cell. Immunology letters. 2007;108:78–87. doi: 10.1016/j.imlet.2006.10.007. doi:10.1016/j.imlet.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 44.Jin HT, Ahmed R, Okazaki T. Role of PD-1 in regulating T-cell immunity. Current topics in microbiology and immunology. 2011;350:17–37. doi: 10.1007/82_2010_116. doi:10.1007/82_2010_116. [DOI] [PubMed] [Google Scholar]
- 45.Ghebeh H, Barhoush E, Tulbah A, Elkum N, Al-Tweigeri T, Dermime S. FOXP3+ Tregs and B7-H1+/PD-1+ T lymphocytes co-infiltrate the tumor tissues of high-risk breast cancer patients: Implication for immunotherapy. BMC cancer. 2008;8:57.. doi: 10.1186/1471-2407-8-57. doi:10.1186/1471-2407-8-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ascierto PA, Simeone E, Sznol M, Fu YX, Melero I. Clinical experiences with anti-CD137 and anti-PD1 therapeutic antibodies. Seminars in oncology. 2010;37:508–16. doi: 10.1053/j.seminoncol.2010.09.008. doi:10.1053/j.seminoncol.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 47.Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH. et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2010;28:3167–75. doi: 10.1200/JCO.2009.26.7609. doi:10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Emens LB, Cassier P, DeLord J-P, Eder JP, Shen X, Xiao Y, Inhibition of PD-L1 by MPDL3280A leads to clinical activity in patients with metastatic triple-negative breast cancer. San Antonio Breast Cancer Symposium. 2014; 2014. PD1-6. [Google Scholar]
- 49.Nanda RC, Dees EC, Berger R, Gupta S, Geva R, Pusztai L. et al. A phase Ib study of pembrolizumab (MK-3475) in patients with advanced triple-negative breast cancer. San Antonio Breast Cancer Symposium. 2014:S1–09. [Google Scholar]
- 50.Gibson J. Anti-PD-L1 for metastatic triple-negative breast cancer. The Lancet Oncology; 2015. doi:10.1016/S1470-2045(15)70208-1. [DOI] [PubMed] [Google Scholar]
- 51.Webster WS, Thompson RH, Harris KJ, Frigola X, Kuntz S, Inman BA. et al. Targeting molecular and cellular inhibitory mechanisms for improvement of antitumor memory responses reactivated by tumor cell vaccine. Journal of immunology. 2007;179:2860–9. doi: 10.4049/jimmunol.179.5.2860. [DOI] [PubMed] [Google Scholar]