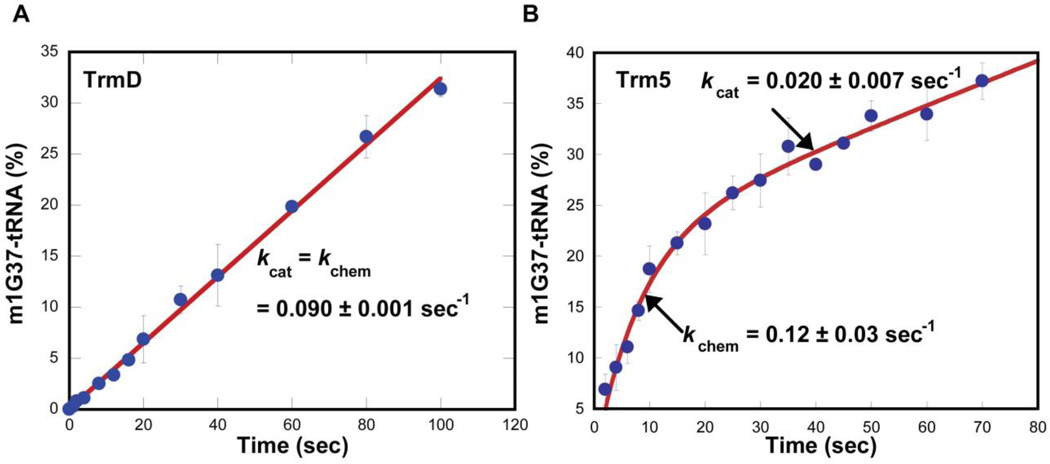
Figure 2.
Pre-steady-state kinetics of m1G37-tRNA synthesis. (A) Monitoring the time course of synthesis upon mixing EcTrmD (1 µM) with EctRNALeu (10 µM) and AdoMet (30 µM) at 37 °C. The time-dependent synthesis is calculated as the amount of synthesis per active site of the enzyme (%) and is fit to equation 1 to determine the slope (a = slope and b = 0). (B) Monitoring the time course of synthesis upon mixing MjTrm5 (1 µM), MjtRNACys (10 µM), and AdoMet (25 µM). The time-dependent synthesis is calculated as the amount of synthesis per active site of the enzyme (%) and is fit to equation 3 to determine kchem and kcat. This figure is adapted from Figure 1 in (Christian et al., 2010b).