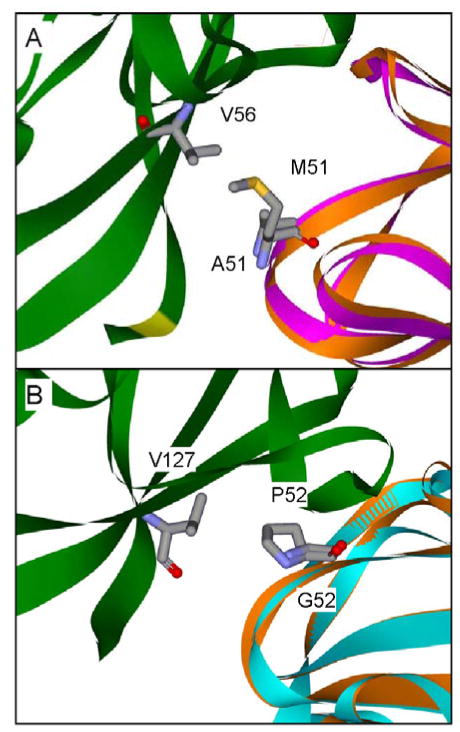
Figure 8.
Interactions involving amicyanin residues Met51 and Pro52 at the MADH-amicyanin interface. Protein backbones of the MADH β subunit (green), native amicyanin (orange), M51A amicyanin (purple) and P52G amicyanin (cyan) are shown as solid ribbons with amicyanin residues 51 and 52, and residues on MADH with which they interact are shown as sticks. Structures (A) M51A amicyanin (PDB, 2QDV)48 and (B) P52G amicyanin (PDB, 2GB2)24 are shown in relation to the MADH structure after alignment with the amicyanin portion of the native complex structure (PDB, 2GC4).