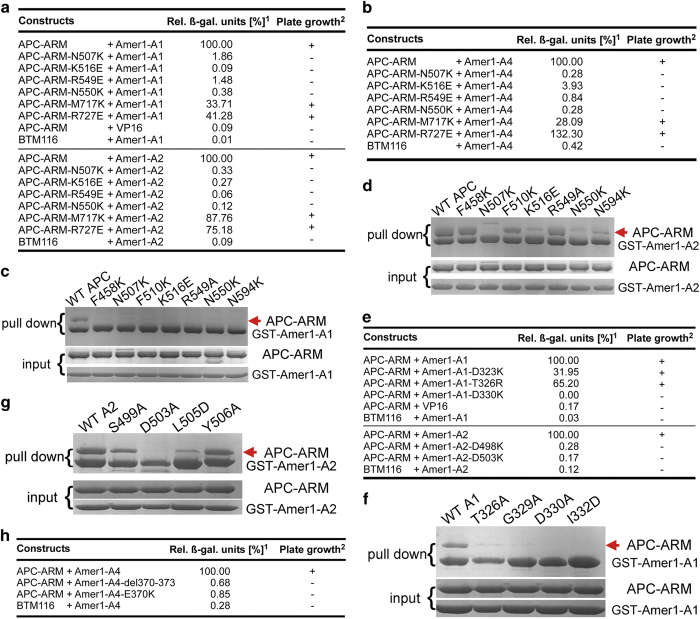
Figure 3.
Mutation of critical APC or Amer1-A1/A2/A4 interfaces residues abrogated the associations of APC–ARM with Amer1-A1, -A2, and -A4. (a, b) Point mutants of crucial interface residues on APC–ARM were defective in recognizing murine Amer1-A1 (residues 279–367) (a), -A2 (residues 388–551) (a), and hAmer1-A4 (residues 337–455) (b) in the yeast two-hybrid assay. 1 Relative β-galactosidase reporter activity. 2 Growth of transformed yeast on –His selective media. Expression levels of WT and mutant APC–ARM constructs used are shown in Supplementary Figure S4. (c, d) Substitutions of key APC–ARM residues abolished its complex formation with hAmer1-A1 (c) and -A2 (d), as demonstrated by the GST pull-down assay. (e) Point mutations of key interface residues on hAmer1-A1 (residues 280–369) or -A2 (residues 380–531) disrupted their interactions with APC–ARM, as shown by the yeast two-hybrid assay. Expression levels of WT and mutant Amer1-A1/A2 constructs used are shown in Supplementary Figure S6. (f, g) hAmer1-A1 (f) and -A2 (g) mutants with key APC–ARM-interacting residues altered had diminished affinities for APC–ARM, as revealed by the GST pull-down assay. (h) Point mutation or deletion of key interface residues on hAmer1-A4 (residues 337–455) abolished its interaction with APC–ARM, as demonstrated by the yeast two-hybrid assay. Expression levels of WT and mutant hAmer1-A4 constructs used are shown in Supplementary Figure S8.