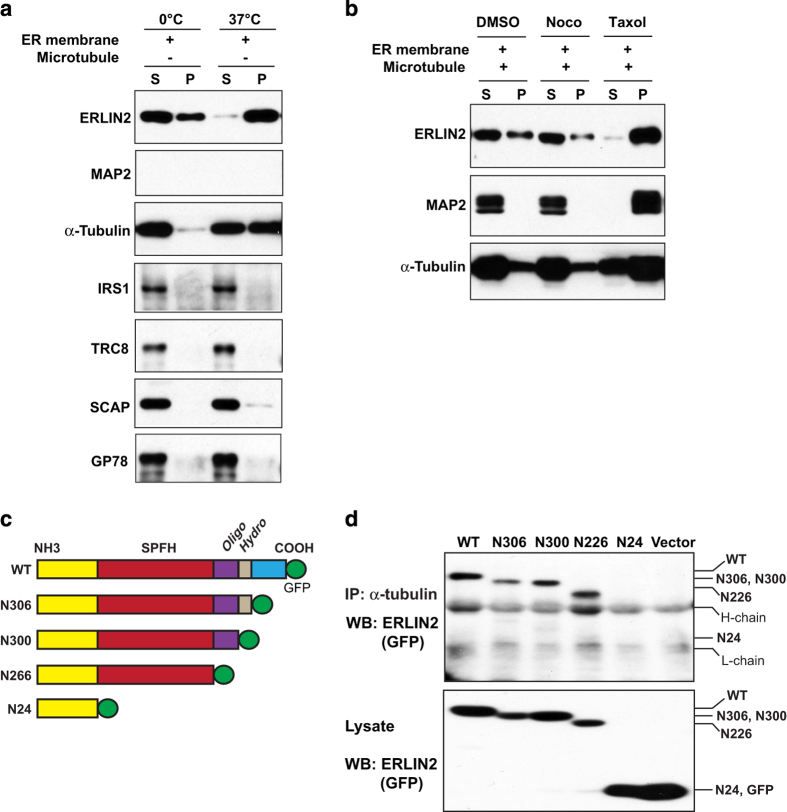
Figure 3.
Endoplasmic reticulum lipid raft-associated protein 2 (ERLIN2) cosediments with microtubules and interacts with α-tubulin via its SPFH domain. (a,b) ERLIN2 co-assembles with microtubules in vitro. ERLIN2-enriched membrane proteins were mixed with mouse brain microtubule extracts at a ratio of 3:1 and then incubated at different conditions (0 °C, 37 °C, nocodazole or taxol treatment) to polymerize or depolymerize the microtubules. The samples were spun through a cushion buffer containing glycerol to collect polymerized microtubule pellets (P) and supernatants (S), which were subjected to immunoblotting analysis using the antibody against MAP2, ERLIN2 or α-tubulin. Levels of MAP2 were determined as a microtubule-associated protein control. (c) Schematic representation of different domains on ERLIN2 protein. SPFH, SPFH domain (22–226 amino acids (aa)); oligo, oligomerization domain (228–300aa); hydro, hydrophobic patch (301–306aa). Plasmid vectors were constructed to express ERLIN2 or its deletion mutations (n306, n300, n226, n24) fused with a C-terminal GFP. (d) Immunoprecipitation (IP)–Western blot analysis of the interaction between GFP-tagged ERLIN2 or various ERLIN2 deletion mutations and α-tubulin in CHO cells. Empty vector (pEGFP-N1 vector) was included as the control. Total cell lysates from transfected CHO cells were immunoprecipated with the anti-α-tubulin antibody. The pull-down proteins were subjected to immunoblotting analysis using anti-GFP antibody. The levels of GFP-tagged ERLIN2 variants in total cell lysates were determined as the controls. H-chain, IgG heavy chain; L-chain, IgG light chain. For a–b and d, the experiments were repeated at least three times, and representative data were presented.