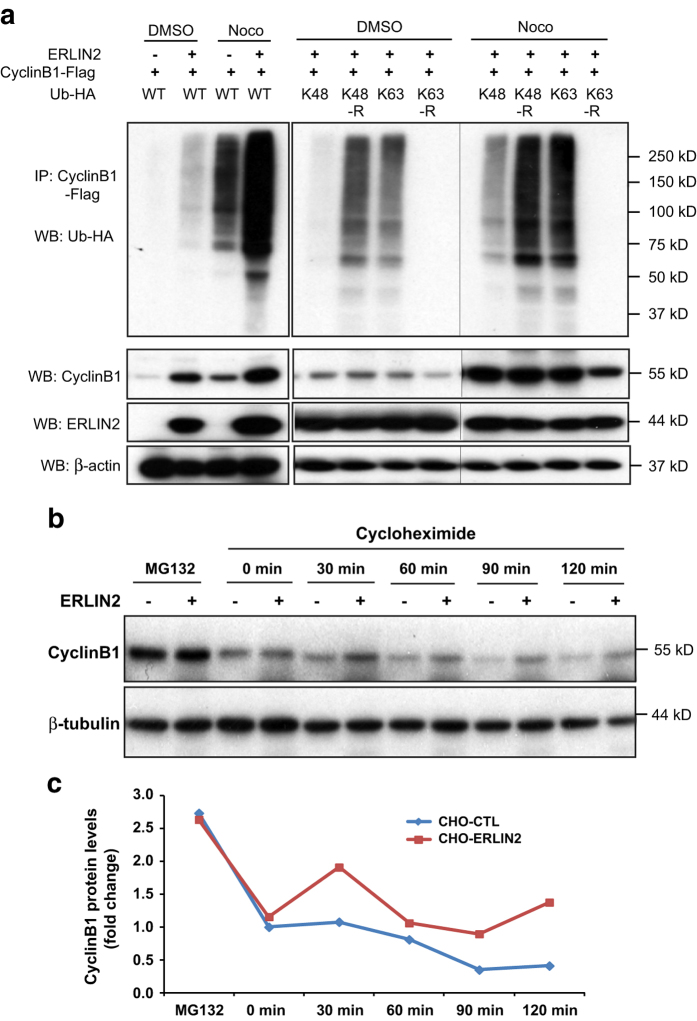
Figure 5.
Endoplasmic reticulum lipid raft-associated protein 2 (ERLIN2) promotes K63-linked ubiquitination of Cyclin B1 and stabilizes Cyclin B1 in G2/M phase. (a) Immunoprecipitation (IP)–western and western blot (WB) analyses of Cyclin B1 ubiquitination and Cyclin B1 protein levels in ERLIN2-expressing CHO cells. Expression vectors for flag-tagged Cyclin B1 and HA-tagged ubiquitin (Ub) or its isoforms, including K48, K63, K48R and K63R, were cotransfected into the CHO cells stably expressing exogenous ERLIN2 or control CHO cells. The CHO cells were then treated with the vehicle dimethyl sulfoxide (DMSO) or Nocodazole to synchronize the cells in the G2/M phase. The cell lysates were immunoprecipated with the anti-flag antibody to pull down Cyclin B1 and then probed with the anti-HA antibody to detect ubiquitination. The lower panel showed the western blot analysis with the protein lysates from the same cells for the levels of Cyclin B1, ERLIN2 and β-actin proteins. (b) Cycloheximide translation inhibition analysis of Cyclin B1 protein stability. ERLIN2-overexpresing (CHO-13) and control CHO cell lines were treated with 10 μg ml−1 cycloheximide and collected at 0, 30, 60, 90 and 120 min after the treatment. Before addition of cycloheximide, cells were arrested in G2/M phase by treating with 120 ng ml−1of nocodazole for 16 h. To confirm that the decrease in Cyclin B1 levels is a result of proteasome-mediated protein degredation, 5 μm of MG132 were added together with nocodazole. The protein lysates collected from the CHO cells were subjected to western blot analysis to determine the levels of Cyclin B1 and β-actin (loading control). (c) Quantification of fold changes in Cycline B1 protein levels in ERLIN2-overexpresing and control CHO cells following cycloheximide treatment. The protein signal intensities, determined by western blot analysis as shown in c, were quantified using Quantity One software. After normlization to β-actin levels, fold changes of Cyclin B1 protein levels were calculated by comparing the Cyclin B1 protein levels to that in MG132-treated CHO control cells (defined as 1). For a–c, the experiments were repeated at least three times, and representative data were presented.