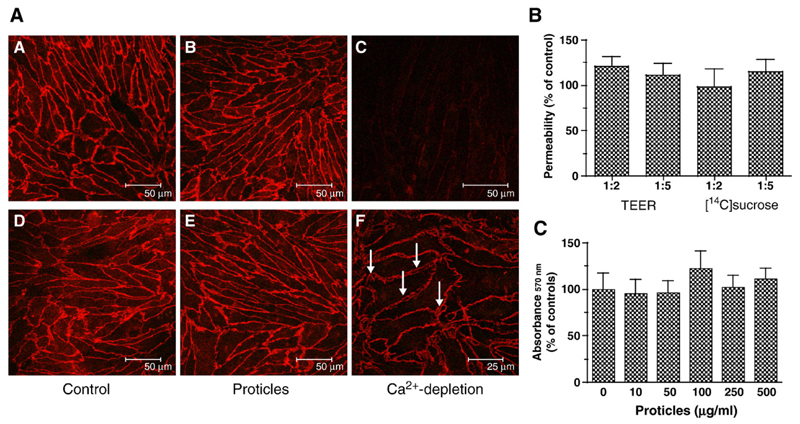
Fig. 1.
Effects of proticles on tight and adherens junctions, transendothelial electrical resistance (TEER) and sucrose permeability, and viability of BCEC. (A) BCEC were cultured on collagen-coated chamber slides as described in Materials and methods, incubated (4 h) in the presence of proticles (2.5 μg ON, ON/protamine mass ratio of 1:5) and processed for immunocytochemistry. Labeling of ZO-1 (A–C) was performed with anti-human ZO-1 antiserum (1:50) followed by Cy-3-labeled goat anti-rabbit IgG (1:300). Immunostaining of VE-cadherin (D–F) was performed with a mouse monoclonal antibody (1:200) and a Cy-3-labeled goat anti-mouse IgG (1:300). Tight junctions were disrupted by Ca2+-depletion in serum-free medium containing EDTA (2.5 mM; arrow in F indicates gap formation between adjacent cells). (B) BCEC were cultured in 12-well Transwell clusters in the presence of proticles (2.5 μg ON/ml; ON/protamine mass ratio of 1:2 and 1:5; 4 h). TEER was measured with an Endohm electrode, [14C]sucrose permeability was assessed as described in Materials and methods. Results are mean±S.D. (n = 4). TEER of controls was 590 ± 125 Ω cm2, [14C]sucrose permeability of control cultures was 1.2 ×10−6 cm/s. (C) BCEC were incubated in the presence of the indicated proticle concentration (ON/protamine mass ratio of 1:5, 4 h), washed, and 50 μl of the MTT solution was added to cells in 500 μl medium. After 1 h, the cells were washed, lysed, and processed for measurement as described in Materials and methods. Results are mean±S.D. (n = 3).