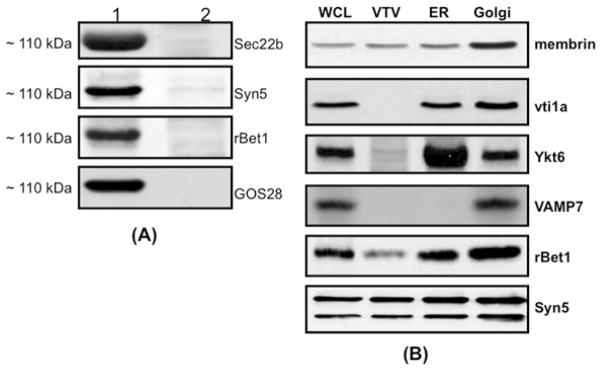
Figure 5. Proteins co-immunoprecipitated with Sec22b in a ~110 kDa complex after VTV–Golgi docking.
(A) VTVs (150 μg) were incubated at 4 °C with hepatic cis-Golgi (300 μg), cytosol (500 μg of protein), and without Mg2+-ATP. VTV–Golgi complexes were isolated on a sucrose step gradient. VTV–Golgi complexes were solubilized in 2 % (v/v) Triton X-100 and incubated with anti-Sec22b antibodies bound to agarose beads at 4 °C overnight. The beads were washed and either not boiled (lane 1) or boiled (lane 2) in Laemmli buffer. The proteins were separated by SDS/PAGE (5–15 %) and probed with antibodies against the indicated proteins. A single membrane was used, which was sequentially probed with the indicated antibodies after washing. Only protein bands that migrated at ~ 110 kDa are shown. Detection was carried out by ECL. The results are representative of four experiments. (B) Samples of hepatic whole-cell lysate (WCL), VTVs, ER and Golgi (each containing 30 μg of protein) were separated by SDS/PAGE (12 % gel), transblotted on to a nitrocellulose membrane and probed with specific antibodies against the indicated proteins. Protein detection was by ECL.