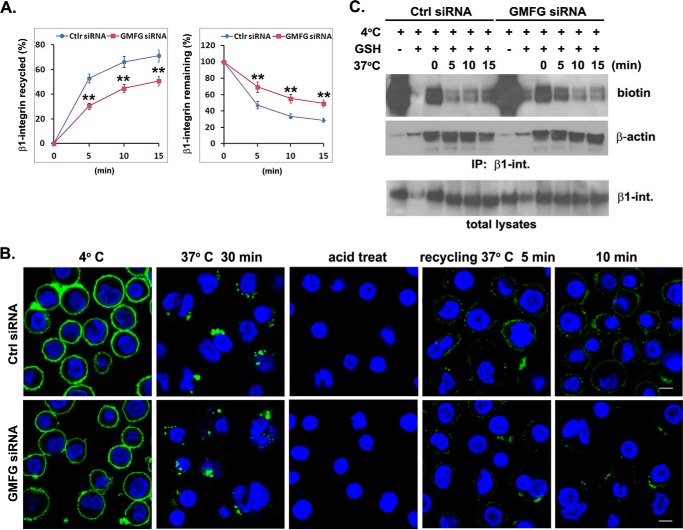
FIGURE 6.
GMFG knockdown inhibits efficient β1-integrin recycling to the cell surface of human monocytes. A and B, human monocytes were transfectedwith control negative siRNA (Ctrl siRNA) or GMFG siRNA. Forty-eight h after transfection, cell surface β1-integrin was labeled with anti-β1-integrin antibody at 4 °C, followed by incubation of cells at 37 °C for 30 min to induce internalization. Cells were then acid-washed to remove the remaining surface-bound labeled integrin antibody, and the internalized β1-integrin was chased back to the cell surface at 37 °C for the indicated time periods. A, antibody-based assay of β1-integrin recycling. Cells were stained with Alexa Fluor 488-conjugated secondary antibody at 4 °C, followed by quantification of surface β1-integrin by flow cytometry. Data represent the mean ± S.D. (error bars) of three experiments. **, p < 0.01 compared with control siRNA-transfected cells. B, immunofluorescence-based assay of β1-integrin recycling. Cells were stained with Alexa Fluor 488-conjugated secondary antibody without permeabilization to examine β1-integrin trafficking back to the cell surface. Nuclear DNA was labeled with DAPI (blue). Scale bar, 100 μm. C, biotinylation-based assay of β1-integrin recycling. Monocytes transfected with control negative siRNA or GMFG siRNA were surface-labeled with 0.5 mg/ml sulfo-NHS-SS-biotin at 4 °C for 1 h, and then internalization was induced at 37 °C for 30 min. Cells were exposed to GSH solution at 4 °C to remove surface biotin and then chased at 37 °C for the indicated time periods to allow recycling, followed by a second reduction with glutathione. Biotinylated cell surface proteins remaining inside the cells were immunoprecipitated using an anti-β1-integrin antibody and subsequently detected by Western blotting analysis using an anti-biotin or anti-β-actin antibody. Samples of the total lysates are shown in the bottom panel; each sample corresponds to 5% of the cell lysate used in each immunoprecipitation. The blots are representative of three independent experiments. β-Actin was used as a loading control.