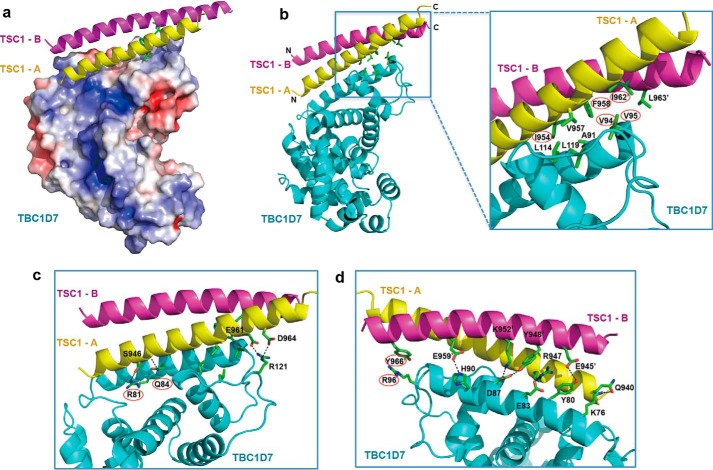
FIGURE 3.
Details of the TSC1-TBC1D7 interface. a, electrostatic potential of the surface of TBC1D7. The core TSC1-binding surface of TBC1D7 is largely hydrophobic. b, hydrophobic interactions stabilizing the TSC1-TBC1D7 interaction. c, polar interactions on the “front” of the TSC1-TBC1D7 interface. D, polar interactions on the “back” of the TSC1-TBC1D7 interface. For clarity, the front interactions and hydrophobic interactions are not shown. Key interface residues (TSC1: Ile954 and Phe958 in the TSC1-A helix, Ile962′ and Tyr966′ in the TSC1-B helix; TBC1D7: Val94, Val95, Arg81, Gln84, and Arg96) are marked with red ovals.