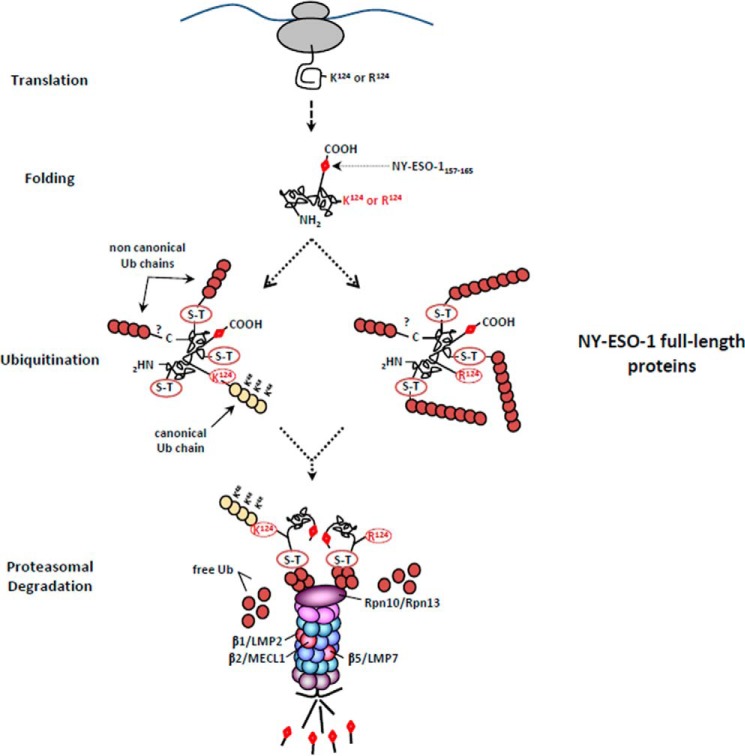
FIGURE 10.
The processing of NY-ESO-1 relies on atypical ubiquitination occurring on non-lysine sites. The presence of the NY-ESO-1157–165 antigenic peptide at the C terminus of the 180-amino acid-long NY-ESO-1 full-length protein required the protein to be fully synthetized before ubiquitination. Following translation, the full-length NY-ESO-1 protein was subjected to both typical and atypical ubiquitination on Lys-124 and on non-canonical sites (Ser-Thr), respectively. The removal of Lys-124 abrogated the formation of Lys-48-linked poly-Ub chains, which was also accompanied by increased generation of atypical chains. Despite these qualitative and quantities differences, both the wild-type and lysine-less forms of NY-ESO-1 converged on the same regulatory proteasome-mediated MHC class I processing pathway involving the Rpn10 and Rpn13 19S subunits as well as the trypsin-like activity of the β2 standard subunit. This eventually leads to similar production of antigenic peptides, which are equally presented on HLA-A*0201 molecules.