FIGURE 3.
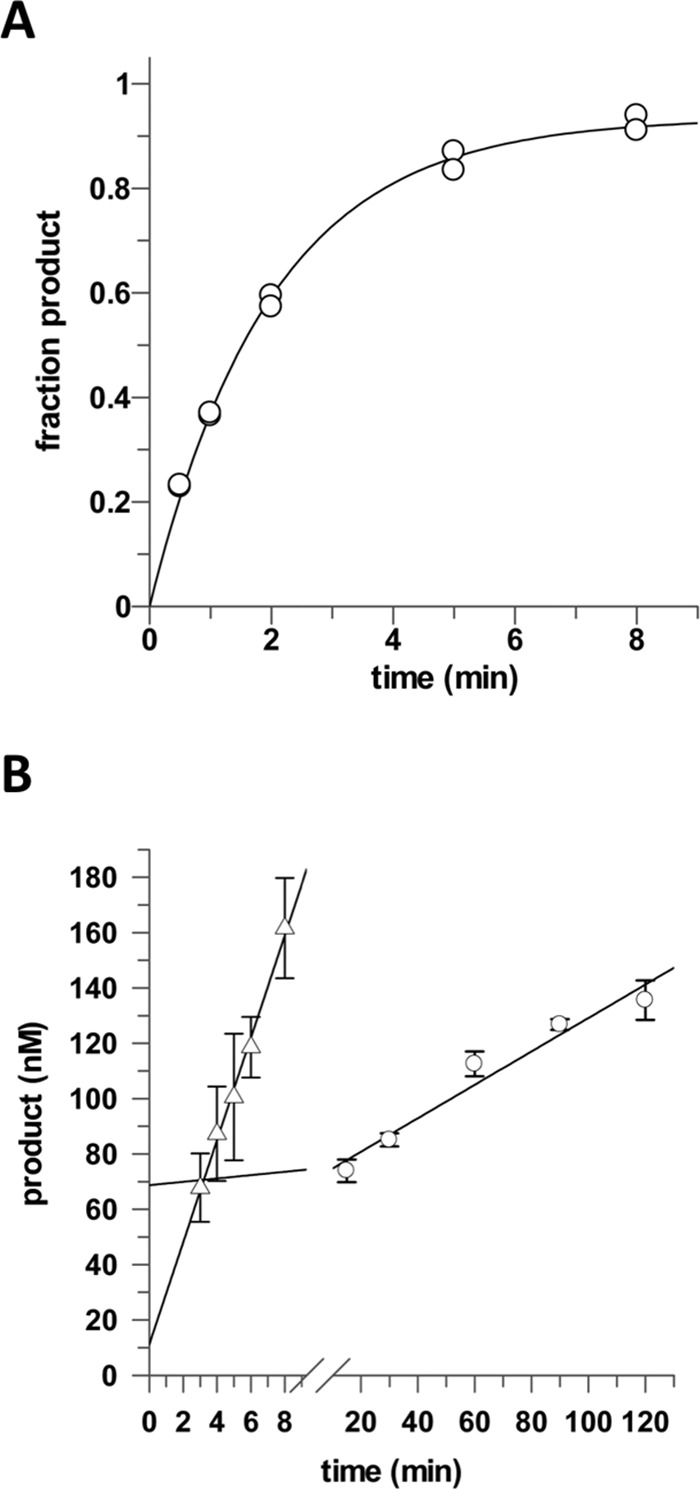
APE1 increases the catalytic turnover of TDG for processing G·caC substrates. A, TDG excision of 5caC from a G·caC DNA substrate exhibits a maximal rate constant of kmax = 0.50 ± 0.02 min−1. Single turnover experiments were performed at 37 °C with 0.5 μm substrate and saturating TDG (1.0 μm). B, catalytic turnover of TDG for a G·5caC substrate and the stimulatory effect of APE1. Multiple turnover activity of TDG is very low in the absence of APE1, kcat = 0.0121 ± 0.0015 min−1 (○), and is dramatically enhanced by APE1, kcat+APE1 = 0.369 ± 0.018 min−1 (▵). Multiple turnover experiments were collected at 37 °C with 1.0 μm DNA substrate, 0.05 μm TDG, and no APE1 or 1.2 μm APE1.
