FIGURE 1.
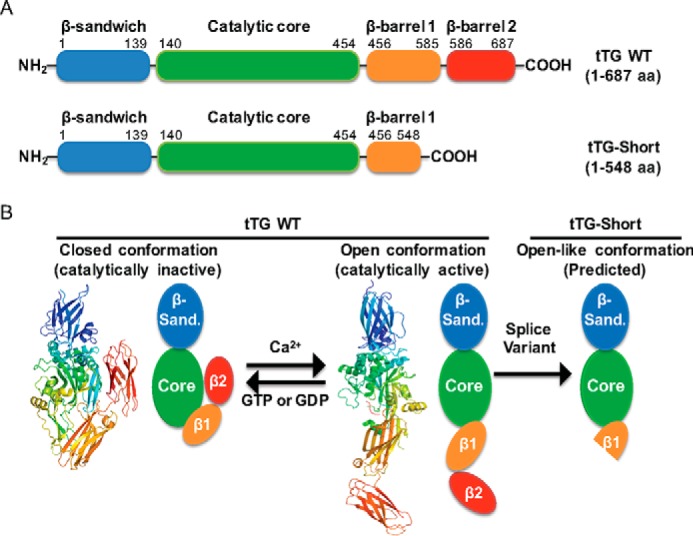
tTG adopts two different conformational states. A, linear representations of tTG WT (top) and tTG-Short (bottom). tTG WT is composed of four domains including an N-terminal β-sandwich (blue) and a catalytic core (green) followed by β-barrel 1 (orange) and β-barrel 2 (red) at its C terminus. tTG-Short is an alternative splice variant of tTG that lacks approximately the last third of β-barrel 1 and all of β-barrel 2. The numbers indicate the amino acids that encode each domain. B, the x-ray crystal structures and simplified diagrams show the closed (catalytically inactive) and open (catalytically active) conformations of tTG WT as well as the open-like conformation that tTG-Short is suspected to assume. The x-ray crystal structure of the closed conformation of tTG WT (far left) represents the GDP-bound form of the protein (PDB code 1KV3), whereas the structure shown for the open state conformation (center) represents tTG WT covalently modified with a substrate-mimetic gluten peptide (PDB code 2Q3Z). GDP and the gluten peptide are shown in sticks and colored in blue and red, respectively. The simplified diagrams show how the different domains in tTG WT are arranged in the closed (far left) versus open (middle) conformations as well as are used to highlight the suspected conformation of tTG-Short (far right). The various domains in tTG are labeled as follows; the N-terminal β-sandwich (β-sand.), the catalytic core (Core), and the two C-terminal β-barrels (β1 and β2).
