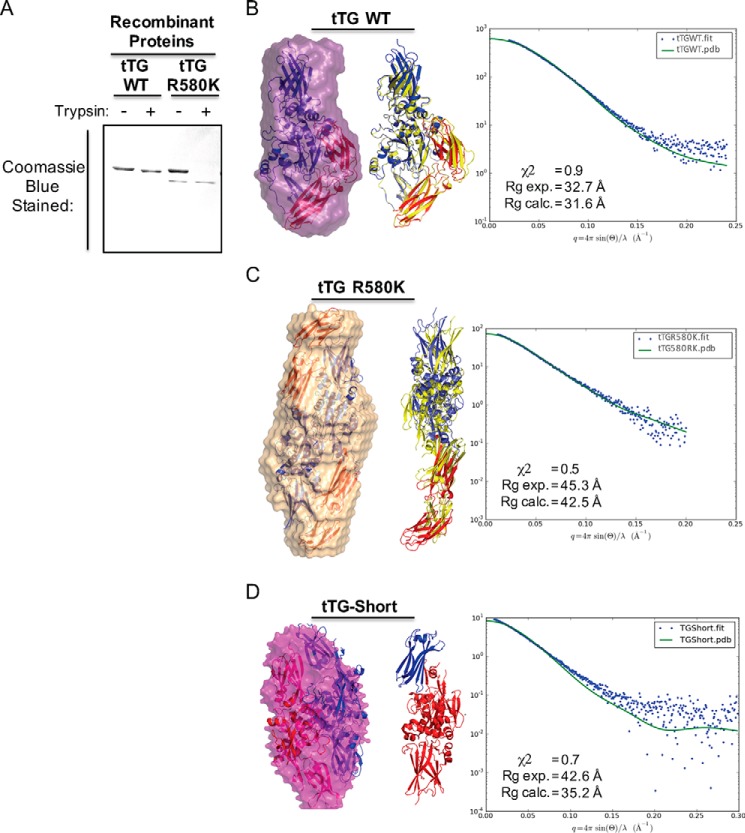
FIGURE 4.
tTG R580K and tTG-Short adopt open conformations in solution. A, purified recombinant forms of tTG WT and tTG R580K (3.5 μg of each) were incubated without or with trypsin for 2 h before being resolved by SDS-PAGE and stained with Coomassie Blue to visualize the proteins. Note that the tTG R580K mutant is more susceptible to trypsin digestion than tTG WT. B, SAXS analysis was performed on recombinant tTG WT. A monomeric model derived from the crystal structure of GDP-bound wild type tTG (PDB code 1KV3) was fitted into the calculated SAXS envelope, with the N-terminal β-sandwich and the catalytic core domains colored in blue and the two C-terminal β-barrel domains colored in red (left panel). A schematic representation shows the superimposition of the fitted model (blue and red) onto the structure of wild type tTG bound to GDP (PDB code 1KV3, yellow) (middle panel). The experimental scattering profiles from SAXS are shown as blue dots, and the scattering profile for the GDP-bound wild type tTG structure (PDB code 1KV3) is shown as a green line (right panel). C, SAXS analysis was performed on recombinant tTG R580K. Two monomeric models derived from a substrate-bound tTG WT crystal structure (PDB code 2Q3Z) were fitted into the calculated SAXS envelope for tTG R580K in a head-to-tail fashion. The N-terminal β-sandwich and the catalytic core domains of the fitted models are colored in blue, and the two C-terminal β-barrel domains are colored in red (left panel). A schematic representation shows the superimposition of one of the monomers in the fitted model (blue and red) onto the structure of the substrate-bound tTG (PDB code 2Q3Z, yellow) (middle panel). The experimental scattering profiles from SAXS are shown as blue dots, and the scattering profile for the substrate-bound tTG WT structure (PDB code 2Q3Z) is shown as a green line (right panel). D, SAXS analysis was performed on recombinant tTG-Short. Two monomeric models derived from a substrate-bound tTG WT crystal structure (PDB code 2Q3Z) were fitted into the calculated SAXS envelope for tTG-Short in a head-to-tail fashion. One monomer is colored in red and the other in blue (left panel). A schematic representation shows one of the monomers in the fitted model. The N-terminal β-sandwich is colored in blue, and the catalytic core domain and the portion of β-barrel that is present in tTG-Short are colored red (middle panel). The experimental scattering profiles from SAXS are shown as blue dots, and the scattering profile for the substrate-bound tTG WT structure (PDB code 2Q3Z) is shown as a green line (right panel).