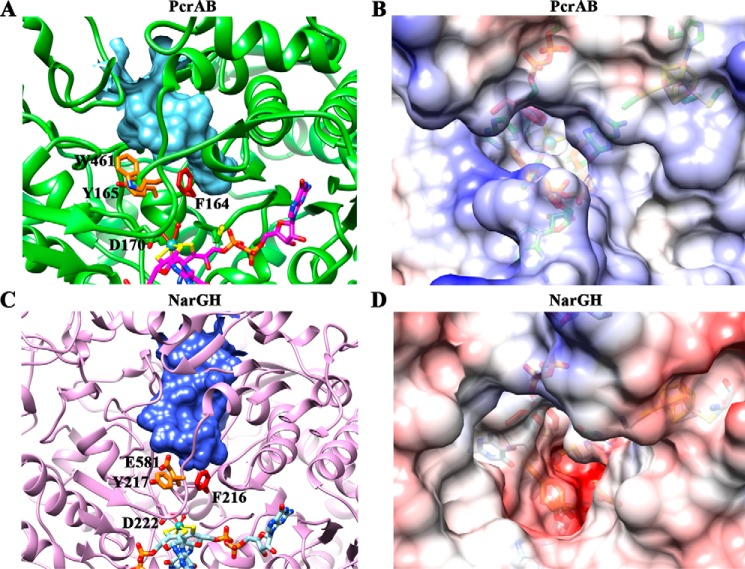
FIGURE 3.
Comparisons of substrate accessing tunnels between PcrA and NarG identify the aromatic gate residues and the differences in electrostatic potential. A, structure of the closed tunnel in PcrA from the surface of the enzyme to the active site as seen in the oxidized PcrAB crystal structure (Fig. 2A) forms a funnel shape (cyan). The surface of the closed tunnel was detected by CASTp (40) and depicted using Chimera (25). The gate residues Phe164 (red), Tyr165 (orange), and Trp461 (orange) are shown in stick. B, the electrostatic potential surface of PcrA. The figure is viewed from the mouth of the tunnel down to the active site. The color is shown from negative (red; −10 kcal/mol × e) through white to positive (blue; 10 kcal/mol × e). C, structure of closed tunnel (blue) in EccNarG (Protein Data Bank code 1Q16) (35) forms a wider tunnel (tunnel volume, 1682 Å3) toward the active site than the tunnel in PcrA (tunnel volume, 1089 Å3). The corresponding gate residues in NarG are shown in stick with the same color code. D, the electrostatic potential surface of NarG by viewing from the same angle as B.