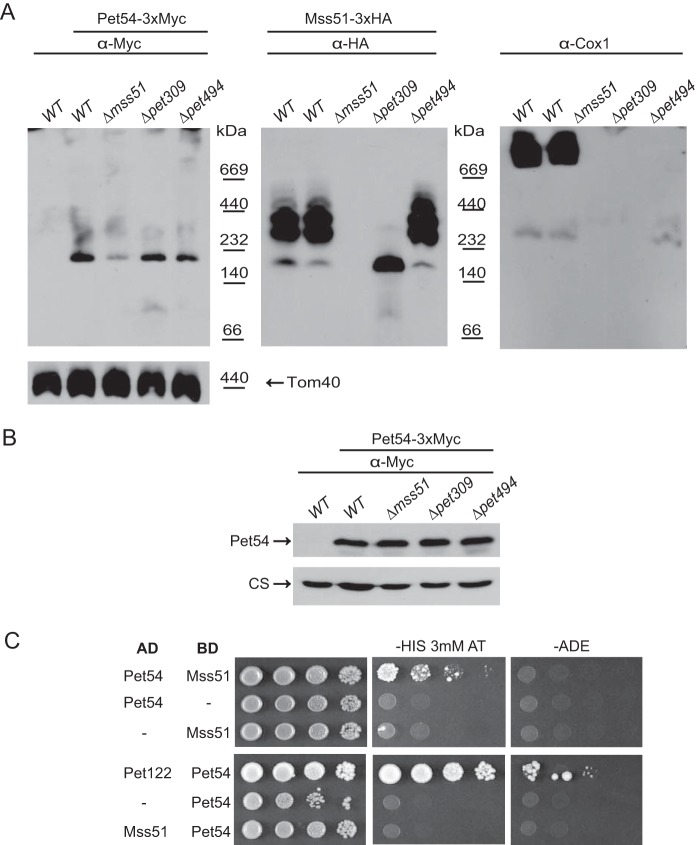
FIGURE 6.
Pet54 migrates into a high molecular weight complex that is different from that of the Mss51 complexes. A, a sample of 100 μg of mitochondrial proteins from cells carrying the Mss51–3xHA and Pet54–3xMyc or the untagged proteins was solubilized with 1% digitonin and separated on a 5–13% acrylamide blue native gel. Western blotting was performed with the indicated antibodies after consecutive antibody-stripping treatments. An antibody against Tom40 was used as a loading control. B, mitochondria from the strains in A were analyzed by SDS-PAGE and Western blotting using antibodies against the c-Myc epitope (to detect Pet54–3xMyc) and citrate synthase as a loading control (CS). C, yeast two-hybrid plasmids containing the Pet54, Mss51, and Pet122 coding regions fused in frame with the activation domain (AD) or binding domain (BD) of Gal4 were co-transformed into the yeast two-hybrid strain Pj69-4a (34) as indicated. The double transformants were selected on medium lacking leucine and uracil or leucine and tryptophan. Growth was tested on medium lacking histidine (in the presence of 3 mm 3-aminotriazole) and medium lacking adenine. 10-Fold serial dilutions were grown at 30 °C for 7 days. Growth of cells containing Pet122-AD and Pet54-BD was used as a positive control of two proteins previously demonstrated to interact (29, 33).