Figure 8.
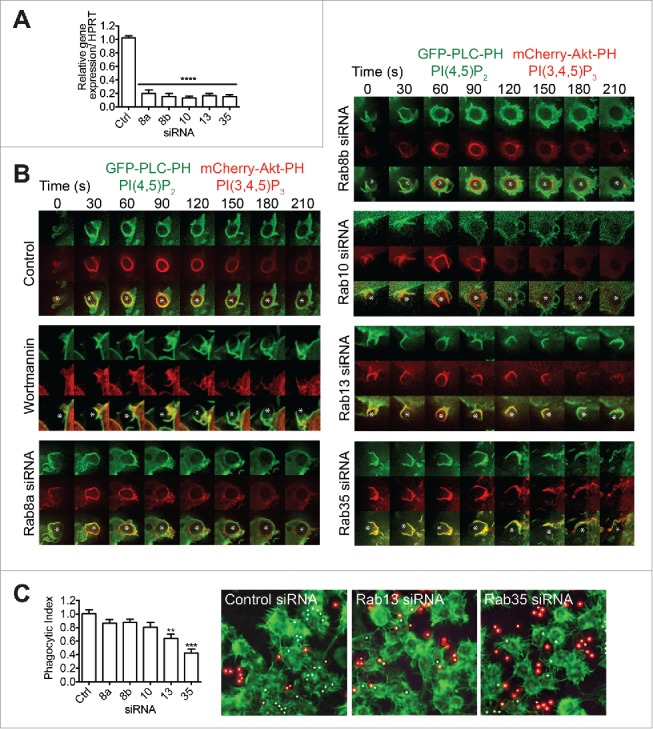
Depletion of early recruited Rabs differentially regulates FcγR-mediated phagocytosis. RAW 264.7 macrophages were treated with pooled siRNAs to target each of the following Rabs - Rab8a, Rab8b, Rab10, Rab13, or Rab35. Cells were siRNA treated for 48h. (A) Quantitative real-time PCR detection of Rab mRNA showing ˜80% depletion of Rab8a, Rab8b, Rab10, Rab13, Rab31, and Rab35 in respective samples. Graph values represent mean ± SEM, n = 3. (B) Cells were cotransfected to express both GFP-PLC-PH and mCherry-Akt-PH. Phagocytosis of IgG-sRBCs (asterisk) was imaged in live cells and representative time-lapse (0–210 s) images are shown. Control cells and cells pre-treated with wortmannin demonstrate normal and disrupted progression of phagocytosis. Cells treated with siRNAs to deplete specific Rabs and then examined in the same regime are then shown. (C) siRNA-treated macrophages were allowed to phagocytose IgG-beads for 15 min then fixed. A phagocytosis assay was used to determine a phagocytic index (proportion of fully internalized beads in each test cell line relative to control). Graph shows mean ± SEM. At least 200 phagosomes from 4 fields of view and 3 individual experiments were quantified. Representative images are shown for knockdown of Rab13 and Rab35. Cells are labeled with phalloidin488 (green); red indicates the externally exposed beads stained with anti-IgG and white only beads are fully internalized
