Abstract
Plasmacytoid dendritic cells (pDCs) bridge innate and adaptive immune responses and have important roles in hematopoietic engraftment, GvHD and graft-versus-leukemia responses following allogeneic hematopoietic cell transplantation (HCT). In addition, pDCs mediate antiviral immunity, particularly as they are the body’s primary cellular source of type I interferon. Given their pleiotropic roles, pDCs have emerged as cells that critically impact transplant outcomes, including overall survival. In this article, we will review the pre-clinical and clinical literature, supporting the crucial roles that pDCs assume as key immune effector cells during HCT.
INTRODUCTION
Allogeneic hematopoietic cell transplantation (HCT) is the definitive cure for many hematologic malignant diseases. However, GvHD, malignant disease relapse and infection remain the primary causes of death following allogeneic HCT.1 Mechanistic understanding of immune cells and associated soluble factors underlying aberrant immune responses is needed to effectively prevent and treat these complications. In this regard, dendritic cells (DCs) have critical roles during allogeneic HCT.2 Specifically, plasmacytoid DCs (pDC) are a distinct subset of DCs that affect innate and adaptive immune responses. This manuscript will review the pre-clinical and clinical literature, supporting the importance that pDCs assume as key immune effector cells during HCT.
OVERVIEW OF DCS: FOCUS ON PDC
Key features of innate immunity include microbial pattern recognition, induction of antimicrobial and immunomodulatory cytokines and chemokines, and instruction of adaptive immunity. DCs have overlapping immune functions as potent APCs for naive T cells, initiation of innate immune response and instruction of subsequent adaptive immune response.3
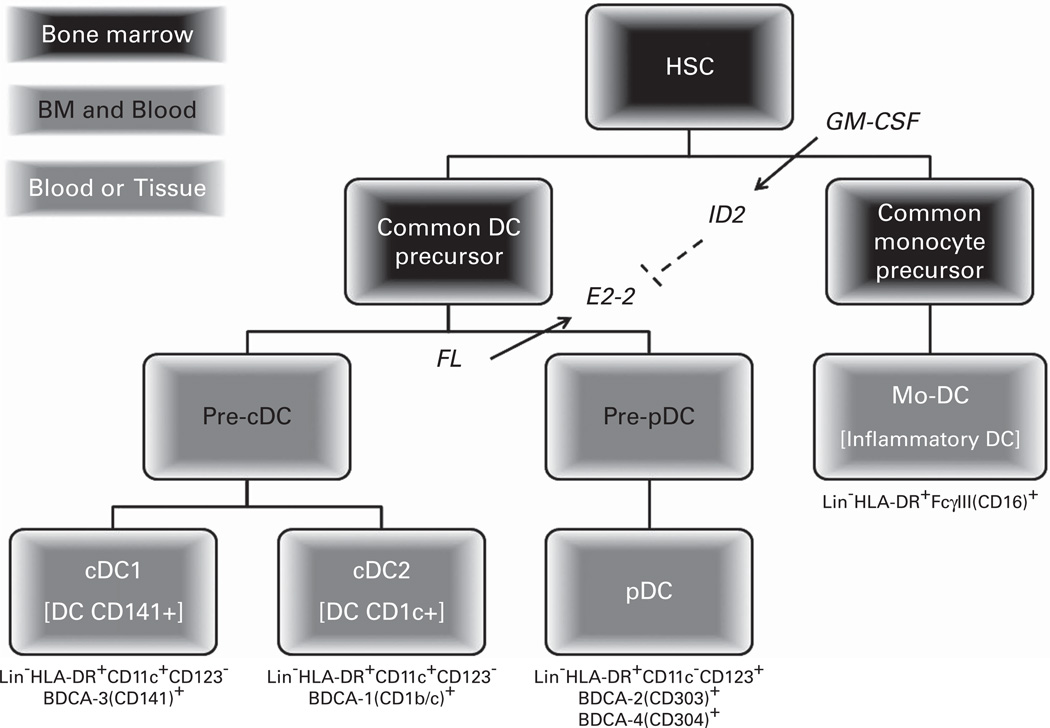
DC classification has changed over the years, reflecting advances in understanding their ontogeny and function. DCs can be broadly categorized into conventional DCs (cDCs) and pDCs4 (Table 1), both of which are derived from precursor DCs (preDCs) that originate from a common DC precursor cell arising from the hematopoietic stem cell (HSC) (Figure 1). Specifically, pDC development requires the transcription factor, E2-2, and the hematopoietic cytokine, fms-like tyrosine kinase 3 ligand (FL).5,6 As absence of FL markedly reduces pDC content in the hematolymphoid tissues7 as does granulocyte-macrophage colony-stimulating factor (GM-CSF)-induced expression of inhibitor of DNA binding 2, a repressor of E2-2.8
Table 1.
Human dendritic cell classification and function
| Conventional DCs | Plasmacytoid DCs | Plasmacytoid precursor DC | |
|---|---|---|---|
| Former names | Classical or myeloid DCs | Interferon-producing cells; lymphoid or plasmacytoid DC2 BDCA-2(CD303)+DC2, CD123+DC2 pDC2=pre-DC2=precursor of type II dendritic cells |
CD8+TCR− FC;127 Plasmacytoid progenitors |
| Subtypes | DC1 =cDC1 =CD141(BDCA-3)+ DC DC2 = cDC2 = CD1c(BDCA-1)+ DC |
CD56dim/−CD3ε+HLA-DR+ CD56brightCD3−εCD11b+CD11c+CD19+ |
|
| General functions |
Ag uptake, processing and presentation IL-12 production enhance CTL and allogeneic T cells |
Steady state: tolerance Activated: Immunogenicity IFNα/β production |
Major constituents of FC population Induce Treg via IL-10, IDO and TGFβ |
Abbreviations: BDCA = blood DC Ag; cDCs = conventional DCs; CTL = cytotoxic lymphocyte; DC = dendritic cell; DC2 = type 2 DC or Th2-inducing DC; FC, facilitating cells; IDO = indoleamine 2,3-dioxygenase; IFN = interferon; IL = interleukin; pDCs; plasmacytoid DCs; p-preDC = plasmacytoid precursor DC; TGF = transforming growth receptor; Treg=T-regulatory cell.
Figure 1.
Human dendritic cell development. Classical (cDC) and plasmacytoid dendritic cells (pDC) derive from a common DC precursor (CDP) cell distinct from monocyte or inflammatory dendritic cells (Mo-DC) that derive from the same common monocyte precursor that macrophages and monocytes arise. Both the common DC and monocyte precursor cells differentiate from common and myeloid progenitor cells (not shown), which arise from hematopoietic stem cells (HSC). Plasmacytoid and monocyte DC development is dependent upon the hematopoietic cytokines, fms-like tyrosine kinase 3 ligand (FL) and granulocyte-macrophage colony-stimulating factor (GM-CSF), respectively. Specifically, FL induces expression of the transcription factor E2-2, which is essential for committing CDP to the pDC lineage. In contrast, GM-CSF induces the transcription factor inhibitor of DNA binding 2 (ID2), which represses E2-2 expression, inhibiting pDC development.
DC activation occurs after recognition of pathogen-associated and danger-associated molecular patterns through pattern recognition receptors known as Toll-like receptors (TLRs). TLRs belong to the TIR (Toll/interleukin-1 receptor) superfamily, which uses a conserved TIR domain in the cytosolic region to activate common signaling pathways.9 The majority of TLRs utilize myeloid differentiation primary response protein 88 as signal adaptor proteins to activate interleukin (IL)-1 R-associated kinases and TNF receptor-associated factor 6, which ultimately activate nuclear factor κB and mitogen-activated protein kinases to initiate synthesis of inflammatory cytokines like IL-6 and TNFα.10 Plasticity and redundancy of cytokine responses directly reflect DC TLR expression.11
Upon activation, cDCs upregulate surface expression of adhesion and costimulatory molecules and change function from Ag-capturing and processing cells to potent APCs that migrate to secondary lymphoid organs and stimulate naive T cells.12 In addition to their roles as APCs, mature cDCs produce cytokines and chemokines, which regulate subsequent innate and adaptive immune responses. For example, cDCs produce IL-12p70, which regulates interferon gamma production in natural killer (NK) cells,13 directs pro-inflammatory T-helper responses14 and enhances DC-NK cell cross-talk.15
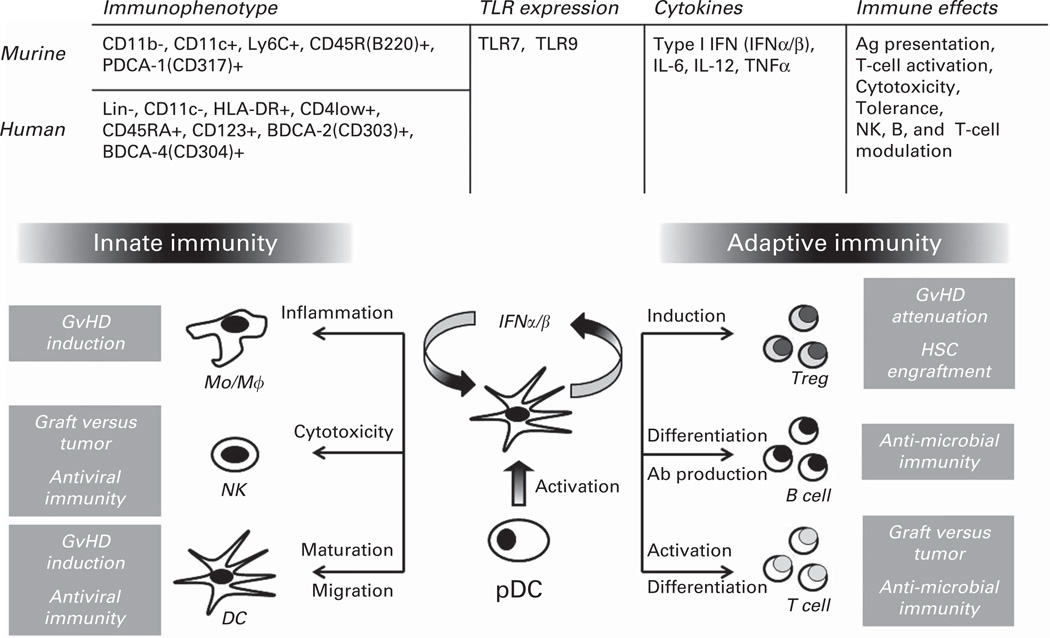
Human pDCs are the principal type I interferon (IFNα/β)-producing cells following infectious challenge.16 Type I IFNs have pleiotropic effects including activating and enhancing NK cytotoxicity and interferon gamma production;17,18 promoting activation, survival and differentiation of Th1 cells;19,20 mediating immune tolerance;21 and potentiating pDC activation itself22 (Figure 2). These effects underlie the critical role that pDC have in supporting antiviral immunity. During the acute phase of RNA (TLR7, ssRNA) and DNA (TLR9, CpG DNA) viral challenge, human pDCs become activated to produce type I IFN, which enhances dendritic, B, T and NK cell function, resulting in viral clearance and generation of memory response. However, pDC type I IFN production can also mediate detrimental effects, including inhibiting viral clearance during chronic infection by modulating APC function to produce IL-10 and to express inhibitory ligands (for example, programmed cell death 1 ligand), which collectively suppress antiviral T-cell function. In addition, type I IFN can increase epithelial cytotoxicity in the host, by enhancing inflammatory monocyte function. Therefore, regulation of type I IFN signaling within pDCs is needed to maintain host homeostasis.23
Figure 2.
Common and distinct characteristics between murine and human plasmacytoid dendritic cells (pDCs) and their putative roles during allogeneic hematopoietic cell transplantation based upon affecting innate and adaptive immune responses. Ab = antibody, Ag = antigen, GvHD = graft-versus-host disease, HSC = hematopoietic stem cell, IFN = interferon, Mo/Mϕ =monocyte/macrophage, NK = natural killer, TLR = toll-like receptor, TNF = tumor necrosis factor, Treg = T-regulatory cell.
Besides their endosomal TLR7 and 9 expression, human pDCs are identified by the following surface immunophenotype: absent CD11c (CD11c−); low-level CD4 (CD4lo+); and high-level CD123bright (IL-3 Rα) and blood DC Ags (BDCA), BDCA-2 (CD303) and BDCA-4 (CD304)24 (Figure 2). Along with these surface proteins, TLR-activated pDCs also upregulate surface co-stimulatory (CD40, CD80 and CD86) receptors that further facilitate their Ag presentation. Activated pDCs also produce cytokines (IL-6, IL-12 and TNFα) that mediate direct effects on pathogens as well as activate immune cells, including macrophages, B-, NK- and T cells. At last, activated pDCs produce chemokines that recruit additional immune cells, further promoting local and systemic immune responses. pDCs also confer tolerogenic effects through cellular and cytokine induction both as activated and inactivated cells.25 For example, pDC have been shown to induce IL-10-producing T-regulatory (Treg) cells, by expressing inducible co-stimulator ligand, which negatively regulates T-cell activation and preferentially drives Treg induction. In addition, pDCs can produce indoleamine 2,3-dioxygenase (IDO), which also inhibits pro-inflammatory T-cell activation and cytokine induction and can also induce Treg cells26 (Figure 2).
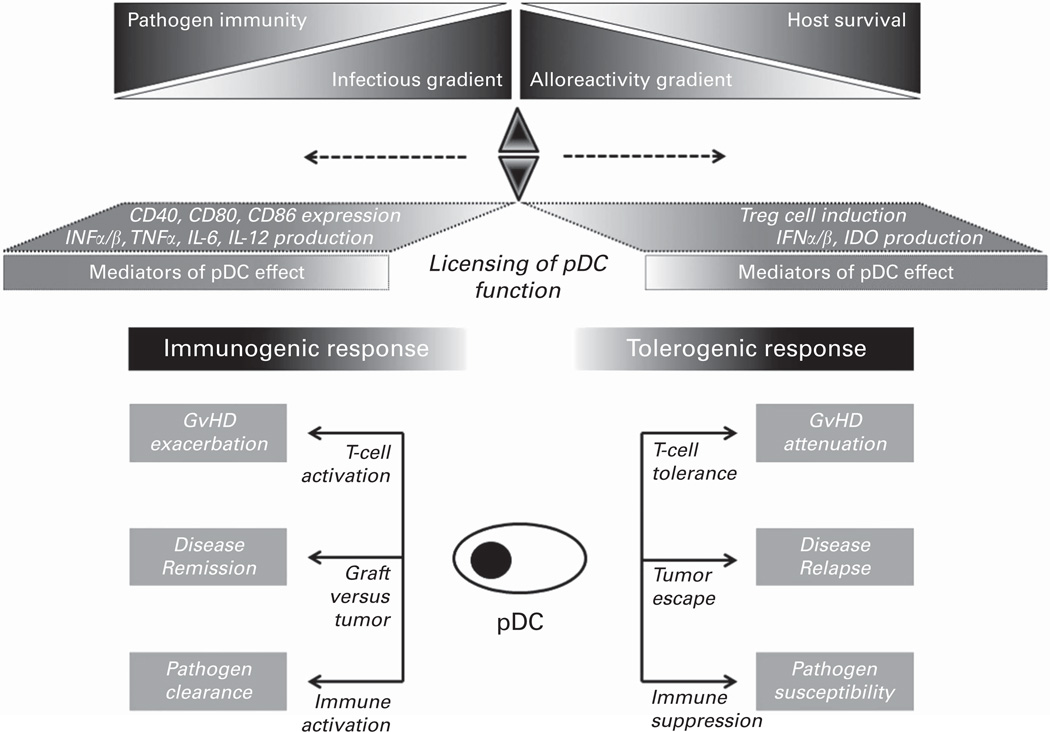
In summary, pDC influence the function of innate and adaptive immune cells and can evoke contrasting responses in the host. Plasticity in pDC function likely reflects both the activation state of pDCs themselves as well as the influence that the micro-environment has in modulating pDC function. Such licensing by the microenvironment may enable pDCs to function differently in complex microenvironments, particularly those associated with allogeneic HCT (Figure 3). This review will summarize the emerging literature describing the multifaceted effects that pDCs have during allogeneic HCT, providing the rationale to pursue novel strategies to expand in number or to modify in function pDCs in order to confer clinical benefit.
Figure 3.
Proposed pDC-mediated immunoregulation in the context of allogeneic HCT. In general, inactivated pDC attenuate T-cell activation through tolerogenic effects mediated by induction of T-regulatory (Treg) cells and production of type I interferon (IFNα/β) and indoleamine 2,3-dioxygenase (IDO). Such tolerogenic effects could be potentially helpful in attenuating acute GvHD, but detrimental in promoting malignant disease relapse and increased risk for infection. In contrast, activated pDC induce T-cell activation via Ag presentation enhanced by surface expression in CD40, CD80 and CD86 as well as production in cytokines (IL-6, IL-12 and TNFα). These immunogenic effects could potentially exacerbate GvHD, but enhance graft-versus-tumor activity and antimicrobial immunity. How pDCs respond in various contexts associated with allogeneic HCT remains undefined. We propose that plasticity in function enables pDC to act as sentinel cells, licensed by the microenvironment to respond to the milieu in which they are found or to which they migrate. In this regard, pDCs may ultimately function as a critical fulcrum, balancing immune surveillance and activation with immune tolerance and modulation.
EFFECTS OF PDC DURING ALLOGENEIC HCT
pDCs have important roles in the setting of allogeneic HCT, including facilitating HSC engraftment, mediating GvL activity and antiviral immunity, and inducing tolerance to attenuate GvHD (Figure 2). The following sections review pre-clinical and clinical studies defining the roles that pDC assume in mediating or affecting post-transplant outcomes, including GvHD, immune reconstitution and infection risk, malignant disease relapse, and patient survival.
HSC AND PDC MOBILIZATION AND ENGRAFTMENT
The HSC niche is a complex microenvironment within the bone marrow comprised of hematopoietic (macrophages) and non-hematopoietic cells (endothelial and perivascular stromal cells, osteoblasts and osteoclasts, CXCL12-abundant reticular cells), sympathetic innervation, and chemical and oxygen gradients.27 HSC mobilization requires cytokines like G-CSF (G) or G in combination with chemotherapy to down regulate CXCL12 expression on stromal cells and to initiate HSC division and emigration from the endosteal to perivascular niche, ultimately leading to increased numbers of HSC and progenitors in the circulation. Specifically, the upregulation of proteolytic enzymes that interfere with CXCR4/CXCL12 or stromal cell-derived factor 1α interactions are critical to enable HSC emigration from the bone marrow (BM).28 In addition to HSC mobilization, hematopoietic cytokines have significant effects on immune cell content within the HSC allograft itself (Table 2). With respect to differences in immune cell graft content between G-CSF- and plerixafor (P) -mobilized grafts, the reader is directed to recent excellent reviews.29–31 A focused review on pDC and HSC mobilization and graft content in allogeneic HCT is provided.
Table 2.
Cytokine and CXCR4 antagonist effects on pDC mobilization and allograft content
| Cytokine(s) | Pre-clinical experience | Clinical experience |
|---|---|---|
| G-CSF | Mobilizes pDC48 | PBSC have more pDC (lin−HLA-DR+CD11c−CD123+) expansion/enrichment than BM32,33,41 |
| GM-CSF | Preferential expansion of myeloid DCs versus pDCs35,36,48 | |
| G+GM | MSD PBSC: fewer pDCs and T cells, higher Th137 | |
| Plerixafor | Induces p-preDC30 | Induces plasmacytoid progenitors (CD34dimCD45RA+ CD123+)71 |
| P+G | Enrichment of conventional T cells, Tregs (CD4+/CD25high/ CD127low/FoxP3+) and pDCs (lin−CD11c−HLA-DR+CD123+) in PB allograft43 Increased pDC content in murine splenocyte grafts128 |
TCRαβ/CD19-depleted haploidentical PBSC grafts are enriched for mDC and pDC42 |
| Flt3 ligand | Expands mDCs and pDCs58,129 and CD8α+DCs | Endogenous FL correlates with DC and NK cell reconstitution56 |
| FL+G | Progenipoietin-1 expands CD8α+DCs,48 mDCs and pDCs103 |
|
| FL+P | Mobilized more pDCs than G alone55 |
Abbreviations: BM = bone marrow; DC = dendritic cell; FL = Flt3 ligand; G = G-CSF; GM = GM-CSF; mDCs = myeloid DCs; MSD = matched sibling donor; NA = unknown/not reported; NK = natural killer; P = plerixafor; PB = peripheral blood; p-preDC = plasmacytoid precursor DC.
G has been shown to mobilize and to expand pDC in the peripheral blood (PB) such that G-mobilized PB stem cell grafts have more pDC (lin−HLA-DR+CD123+CD11c−) than BM grafts,32,33 which may contribute to the comparable incidence of acute GvHD (aGVHD) between G-PB and BM grafts.34 GM-CSF also mobilizes DC, but preferentially expands myeloid DCs (mDCs) versus pDCs35,36 as evidenced by GM+G-mobilized HLA-matched related donor (MRD) PB allografts having fewer pDCs (lin−HLA-DR+CD11c−CD123+) and T cells with higher Th1 content than G-mobilized allografts.37 Furthermore, in HLA-matched unrelated donors (MUD) BM allografts and G-mobilized MRD PB allografts, myeloid and plasmacytoid DC content correlate with CD34 dose, but not DC recovery following allogeneic transplant.38
P alone or in combination with G also mobilizes pDC in PB. However, unlike G-CSF,39–41 P does not polarize DC and T-cell function or expand myeloid cells in the graft.42 In addition to inducing pDCs, P significantly increases T effector memory (Tem) and Treg cells,42–44 both of which have been shown to attenuate aGvHD without compromising GvL.45,46
FL significantly enhances pDC mobilization and expansion in mice47,48 and in humans.6,49 Experience using FL in the allogeneic HCT setting is largely limited to pre-clinical studies in which FL has been shown to modulate GvHD50,51 and to augment thympoiesis and T-cell reconstitution52 to confer protective antiviral immunity53 in mice and to enhance donor HSC engraftment in dogs.54 Of interest, comparing PB murine LSK (Lin−Sca-1+c-kit+) HSC mobilization following administration of G alone, FL alone, P alone, FL+P and G+P, He et al.55 recently showed that FL+P mobilized the most LSK cells, which produced more colony-forming units and expanded the greatest numbers of PB NK, Treg and conventional and plasmacytoid DCs. In addition, FL+P grafts significantly enhanced engraftment in both syngeneic and allogeneic PBSC transplant-recipient mice, as animals receiving FL+P grafts had the highest long-term survival rates among transplant recipient mice receiving PBSC mobilized using alternative cytokines. Results from these pre-clinical studies suggest that FL will have novel effects in the clinical allogeneic transplant setting for both donor-derived hematopoiesis and transplant recipient outcomes including GvHD, infection and malignant disease relapse, especially given that endogenous FL has been shown to correlate with DC reconstitution following allogeneic HCT.56
FL alone or in combination with G or GM-CSF induces generation of tolerogenic DC and Th2-cells in mice47,48,57 and humans,58 which may further contribute to the regulatory effects of pDCs. In addition, G-CSF modulates donor T-cell function by promoting Th2 differentiation through myeloid cell cytokine (IL-4 and IL-10) and pDC induction.32,41,59,60 Similarly, P induces Treg cells,43 but without altering T-cell phenotype and cytokine gene expression like G-CSF.61
Recombinant human FL alone49 or in combination with perixafor55 is emerging as an alternative HSC mobilization regimen to G following chemotherapy. Given its NK and DC induction, recombinant human FL may offer a novel means to enhance both HSC and pDC expansion and to augment innate immune recovery and associated antiviral and antitumor response. Future results from a current clinical trial using CDX-301 (recombinant human FL) alone or in combination with P to mobilize MRD HSCs may substantiate this intended benefit in allotransplant recipients (NCT02200380).
Like mobilization, HSC homing, localization and re-entry of donor hematopoietic progenitors back into the BM (engraftment) is dependent upon soluble factors like CXCL12/stromal cell-derived factor 1α and very late Ag 4 and cellular factors,62 including Treg cells.63 Along with Treg cells, a small CD8+TCR−CD11b−CD11c+B220+ BM population in mice has been identified as engraftment facilitating cells (FCs).64,65 Specifically, plasmacytoid precursor DCs (p-preDCs) comprise the main FC population and share properties with pDCs themselves, including type I IFN and TNFα production, activation by TLR9 ligand (CpG) and FL-induced expansion.66 Mechanistically, p-preDCs and FCs have been shown to promote HSC engraftment following murine allogenic bone marrow transplantataion (BMT) through Treg induction,67,68 which may also explain the reduction in aGvHD severity seen in HSC+FC recipient mice.65,69 FCs may also promote HSC survival via TNFα production; as FCs from TNFα-deficient mice fail to promote engraftment or HSC survival.70
Expanding p-preDCs via cytokine mobilization could potentially facilitate or enhance HSC engraftment in the allogeneic HCT setting. In this regard, P administered to healthy donors and lymphoma patients preferentially mobilized the CD34dimCD45RA+CD123+ subset, which was further characterized as p-preDC.71 Interestingly, the CD34dimCD45RA+ population significantly over-expressed CXCR4 and late Ag 4.72,73 Whether the human plasmacytoid progenitor population functions like its murine p-preDC counterpart with respect to facilitating engraftment and enhancing tolerance remains undefined.
ACUTE AND CHRONIC GVHD
Host DC are critical APCs-initiating aGvHD via direct host Ag presentation,74,75 whereas donor APCs present host Ag to donor T cells via indirect host Ag presentation.76 pDCs can initiate GvHD through radiation-induced MHC II expression, thereby maturing pDCs for T-cell priming.77 In this model, MHC-expressing pDCs were infused into MHC-deficient mice after receiving radiation. MHC II expression was required as it enabled cognate pDC-T-cell interaction, resulting in alloreactive T-cell activation. In contrast, depletion of host cDCs and pDC does not attenuate GvHD, suggesting that host APC-directed approaches alone for preventing GvHD alone will not be successful and inferring that other APC types and/or Ag presentation itself will require targeting.78 Furthermore, although host-type pDC can initiate GvHD under appropriate inflammatory stimuli, it is expected that myeloablative (MA) conditioning regimens will typically deplete host pDC prior to the infusion of donor T cells.
Interestingly, patients with gastrointestinal aGvHD had higher levels of Th17 cells and more RORγt expression in their intestinal mucosa than patients without gastrointestinal aGvHD.79 Furthermore, pDCs were increased in the aGvHD patient’s intestinal mucosa, and their numbers correlated with severity of histologic grading. Together, these findings suggest that pDCs are recruited into GvHD target tissues, wherein they can promote Th17 cytokine production or differentiation or promote Th17 response through IFNα production.80,81 How pDCs actually traffic to gastrointestinal sites of GvHD-associated injury is likely mediated by chemokine receptor expression. Using a model of cholera toxin-induced gut inflammation, Wendland et al.82 showed that CCR9 expression was critical for pDC mobilization to sites of inflammation-induced intestinal injury. In the context of aGvHD, CCR9-expressing cells migrated from secondary lymph tissues to the gut via the CCR9 ligand, CCL25, where they attenuated GvHD.83 Further characterization revealed that these CCR9+ cells were immature pDCs that inhibited T-cell proliferation and cytokine production and induced Treg cells.
pDCs induce Tregs from naive T cells both within primary and secondary lymph tissues,84–87 which attenuate and prevent aGvHD in the pre-clinical88 and clinical89 settings. Therefore, pDC content would seemingly modulate GvHD incidence and severity (Table 3). Several pre-clinical studies have demonstrated the protective effect of donor-derived pDC in attenuating GvHD in transplant recipients. Banovic et al.90 showed that pDC depletion from BM grafts exacerbated GvHD and that reconstituting donor precursor pDCs (CD11clow/PDCA-1+ or CD11clow/120G8+) suppressed allogeneic T cells. Like Banovic et al., Waller and colleagues demonstrated that donor-derived, p-preDCs (lin−CD11b−CD11c+B220+PDCA-1+) diminished GvHD in recipient mice.91,92 Such a protective effect of donor and graft pDCs has also been noted in the clinical transplant setting. Increased pDC cells in MRD BM allografts associated with less-chronic GvHD (cGvHD) in HLA-matched sibling BM recipients.93,94 Similarly, recipients receiving MUD BM allografts with higher pDC content had higher overall survival (OS) due to fewer deaths from GvHD and graft rejection.95
Table 3.
Transplant outcomes based upon pDC graft content and immune recovery following allogeneic hematopoietic cell transplantation
| Outcome | Clinical experience |
|---|---|
| Graft failure aGvHD | TCRαβ/CD19-depleted haploidentical PBSC grafts (P+G): 4/23 primary graft failures130 MSD PBSC (G): low DC1+ pDC (lin−CD123+) (< 4.97/µL) at engraftment, higher risk131 MUD allografts: low D21 pDC, higher incidence aGvHD126 MRD PBSC (G): low D28 PB pDC (⦤4.5/µL) higher risk aGVHD132 TCRαβ/CD19-depleted haploidentical PBSC grafts (P+G): 2/23 patients with skin Grade I/II only130 Lower post-transplant pDC recovery, higher risk severe aGVHD97 Persistence in host DC chimerism at D100 correlates with aGvHD severity100 GvHD histologic grading correlates with pDC content in intestinal mucosa in GI GvHD patients79 |
| cGvHD | MRD BM: higher pDC cell content, lower risk93,94 TCRαβ/CD19-depleted haploidentical PBSC grafts (P+G): No cGvHD130 MRD PBSC (G): low D28 PB pDC (⦤4.5/µL) higher risk cGVHD132 Patients with cGvHD have elevated PB donor-derived pDCs101 |
| Relapse | MRD BM: higher pDC cell content, higher relapse risk94 MRD PBSC (G): higher graft pDC content (⦥2.3 M/kg), higher risk of relapse98 MSD PBSC (G): low DC1+ pDC (lin−CD123+) (< 4.97/µL) at engraftment, higher risk of relapse131 Low D100 pDC level (< 0.2% PBMC), higher relapse122 |
| Immune recovery | MUD BM: higher CD34 content did not correlate with pDC recovery38 MRD PB (G+GM): lower pDC graft content, faster T-cell recovery37 MUD allografts: low D21 pDC associated with poor prognosis reflected by higher NRM and shorter OS126 pDC (linCD11c−CD123+) content is higher in PBSC grafts and correlates with D30 and D100 PB pDC levels114 Endogenous levels of FL correlated with pDC recovery56 Lower post-transplant pDC recovery, higher risk severe aGVHD97 GvHD impedes PB pDCs recovery96,97,114,116,120–122 Steroids reduce PB pDCs116,119,124,133 Steroids118–120,124 and CSA56,118,125 impair pDC function |
| Survival | MUD BM: higher pDC content, increased OS due to reduced TRM (fewer GvHD and graft rejection deaths)95 MRD BM: higher pDC cell content, lower EFS94 MRD PBSC (G): higher graft pDC content (⦥2.3 M/kg), lower OS and EFS98 MSD PBSC (G): low DC1+ pDC (lin−CD123+) (< 4.97/µL) at engraftment, higher death131 MUD allografts: low D21 pDC associated with poor prognosis reflected by higher NRM and shorter OS126 RIC MRD PBSC (G): Low D30 PB pDC, higher TRM, lower EFS and OS134 RIC MRD PBSC (G) & BM: D90 ‘High’ PDC recovery profile (≥0.725/µL), higher OS117 Low D100 pDC level (< 0.2% PBMC), lower OS122 |
Abbreviations: aGvHD = acute GvHD; BM = bone marrow; CSA = cyclosporine; cGvHD = chronic GvHD; EFS= event-free survival FL = flt3 ligand; G = G-CSF; GM = GM-CSF; M = million; MSD = matched sibling donor; MUD = matched unrelated donor; NRM = non-relapse mortality; OS = overall survival; P = plerixafor; PB = peripheral blood; RIC = reduced-intensity conditioning; TRM = transplant-related mortality.
In addition to the protective roles of pDCs in the donor graft in attenuating GvHD, post-transplantation reconstitution of pDCs is predictive for subsequent GvHD risk. Following MA pediatric allogeneic SCT, Horvath et al.96 noted that patients with aGvHD had significantly lower numbers of circulating myeloid and plasmacytoid DCs than non-GvHD patients. Furthermore, the decrease in PB DC numbers preceded the onset of clinical symptoms by at least 24 h and was independent of steroid administration. Low numbers of post-transplant myeloid and plasmacytoid DC in the blood were also associated with the development of aGvHD in another pediatric HCT population receiving MA and either matched sibling donor or MUD allografts.97 Rajasekar et al.98 found that D28 PB pDC predicted incidence of acute and cGvHD following MRD PBSCT. Interestingly, DC chimerism as a predictor of GvHD has also been studied. Pihusch et al.99 found no significant correlation between GvHD and PB DC chimerism, though patients with aGvHD had more mixed pDC chimerism than patients without aGvHD. Similarly, Chan et al.100 noted that persistence of host-type DCs at D100 correlated with the development of severe acute and cGvHD. In contrast, Clark et al.101 found that patients with cGvHD had elevated donor PB pDC versus those recipients without cGvHD. Disparities between these studies regarding pDC origin and content post-transplant may be explained by the analysis of transplant patients receiving predominantly reduced-intensity conditioning (RIC) in the Chan study, whereas patients received MA in the Clark study.
Given their influential roles in mobilizing DCs and T cells and in modulating their expression and function, cytokines used for HSC mobilization may affect GvHD induction and severity. For example, G-CSF modulates donor T-cell function by promoting Th2 differentiation through myeloid cell cytokine (IL-4 and IL-10)59,60 and pDC induction.32,41 In contrast to G-CSF, P induces Treg cells,43 but without altering T-cell phenotype and cytokine gene expression.61 Based upon general DC expansion, FL would seemingly have a complex influence on aGvHD risk. In this regard, Blazar et al.51 found that FL therapy expanded splenocyte myeloid and plasmacytoid DCs and reduced T cells in donors, but did not modify subsequent aGvHD induction when donor splenocytes were administered to transplant recipients. In contrast, transplant mice receiving post-transplant FL succumbed from augmented aGvHD, suggesting that FL had no effect on T-cell phenotype or alloreactivity in donor and upregulated inflammatory cytokines (TNFα) in transplant recipients.51 However, Teshima et al.50 found that FL administered to transplant recipients prior to BMT reduces aGvHD by attenuating early donor T-cell expansion and activation to host alloantigens. In ex vivo experiments, the authors determined that FL expanded ex vivo CD8α+ DCs, which were poor stimulators of allogeneic T cells. Progenipoietin-1 (ProGP-1), a synthetic G-CSF/FL agonist, has also been studied for its effects on aGvHD following murine allogeneic BMT. Comparing effects between donor ProGP-1 and G-CSF pre-treatment and subsequent GvHD in recipient mice, MacDonald et al.102 found that ProGP-1 or G-expanded donor splenocytes co-infused with BM cells into recipient mice resulted in 90% versus 50% survival, respectively. Enhanced survival associated with reduced TNFα induction and gastrointestinal aGvHD in recipients. The ProGP-1 effects were not mediated by donor pDCs, but rather its effects on donor T-cell Ag response and cytokine production. Further interrogation into ProGP-1 effects on donor APCs (that is, its effect on indirect host Ag presentation) revealed that ProGP-1expanded granulocyte/ monocyte precursors, which prevented GvHD through an IL-10-mediated process, whereas myeloid and plasmacytoid DCs augmented GvHD.103
SEPARATING GVL FROM GVHD
Like other DC subtypes, pDCs influence GvL activity following allogeneic HCT.104 First, pDCs produce type I IFN, which has direct effects on tumor cells as well as modulates antitumor immunity.105 Second, in contrast to mDC cells, pDC (lin−HLA-DR+CD11c−CD123+) induce Tregs,106 which can attenuate GvHD and preserve GvL activity. Third, precursor p-preDCs promote Th1/ type 1 cytotoxic lymphocyte differentiation, enhancing GvL activity without increasing GvHD.91,92,107 Finally, hematopoietic cytokines like FL synergize with DC survival and activation cytokines like CD40 ligand to augment antitumor response.108
Given these properties, increasing pDC allograft content could potentially impact the separation of GvHD from GvL (Table 4). In this regard, type I IFN has recently been shown to impact both GvHD and GvL response following murine allogeneic BMT.109 Specifically, type I IFN protected against CD4-dependent GvHD, as it suppressed both ex vivo and in vivo CD4+ T-cell proliferation and differentiation when administered to recipient mice during conditioning. In contrast, type I IFN paradoxically increased CD8-mediated aGvHD without modulating CD8+ T-cell function and post-BMT cytokine administration eradicated low-level tumor burden in recipients. As type I IFN signal through STAT1,110 Capitini et al.111 utilized STAT1-deficient mice as BM donors to determine the effects of STAT1 deficiency on subsequent GvHD and GvL. Recipients of STAT1KO BM had decreased GvHD, preserved GvL activity and enhanced OS compared with transplant recipients receiving WT BM. Furthermore, STAT1KO recipients had increased pDCs, which when depleted post BMT, reversed the protective effect associated with STAT1 deficiency in modulating GvHD. STAT1-deficient, expanded pDCs produced less IFN and more IL-10 via STAT3 induction.
Table 4.
Mechanistic pre-clinical studies addressing the influence of pDC on GvHD and GvL
| Contributory or neutral role | Protective role | |
|---|---|---|
| aGvHD | XRT-activated recipient-type pDC prime alloreactive donor T cells and cause aGvHD77 ProGP-1-expanded host pDCs augment GvHD103 Depletion of host cDCs and pDC does not attenuate GvHD78 Increased pDC in G+P mobilized donor splenocyte grafts increased aGvHD clinical scores and intestine pathology scores in allogeneic recipient mice128 |
p-preDC induce Tregs and reduce aGvHD65,69 BM pDC attenuate aGvHD90 p-preDC attenuate aGvHD90–92 CCR9+ immature pDCs attenuate GI aGvHD83 Pre-transplant FL given to BMT recipients reduces aGvHD via CD8α+ DC expansion50 pDC (lin−HLA-DR+ CD11c− CD123+) induce Tregs106 Type I IFN protects against CD4-dependent aGvHD109 |
| GvL | p-preDCs promote Th1/type 1 CTL differentiation, enhancing GvL activity without increasing GvHD91,92,107 |
Recipients of STAT1KO BM had increased pDCs, decreased GvHD, preserved GvL activity and enhanced OS compared with transplant recipients receiving WT BM111 |
Abbreviations: aGvHD = acute GvHD; Allo = allogeneic; BM = bone marrow; CTL = cytotoxic T lymphocyte; FL = flt3 ligand; G = G-CSF; GM = GM-CSF; M = million; p-preDC = plasmacytoid precursor DC; P = plerixafor; PB = peripheral blood; ProGP-1 = progenipoietin (FL+G); XRT = radiation.
The pDC content in MRD BM,94,98 but not MUD PB or BM,95 allografts has been shown to increase the risk of disease relapse following allogeneic HCT. Given these observations, the Waller laboratory has investigated the effect of manipulating donor APC content as a novel strategy to enhance GvL without exacerbating GvHD during experimental allogeneic BMT. Initial experiments incorporating CD11b depletion from donor BM resulted in enhancement in GvL without increasing GvHD.112 Specifically, recipients of CD11b-depleted BM had significant expansion in splenic donor CD4+ memory T cells compared with recipients of unmanipulated BM, which was proportional to the number of CD11b− DCs in the BM graft and associated with increased levels of interferon gamma. Subsequent experiments focused on identifying the cell source within the donor CD11b− APC population mediating these effects and defining its underlying mechanism. Donor CD11b− APCs were identified as pDC progenitors (lin−CD11b−CD11c+PDCA-1/CD317+) that upregulated CD80/86 and IL-12 during alloAg presentation in contrast to CD11b+ APCs that upregulated programmed death ligands-1 and 2 after activation.91 Transplanting FACS-purified donor pDC progenitors with purified HSCs and congenic T cells induced Th1 donor cytotoxic lymphocyte polarization, enhancing GvL without increasing GvHD in both MHC and minor histocompatibility Ag-mismatched models. In contrast, transplantation using CD11b+ APCs led to donor Th2/type 2 cytotoxic lymphocyte polarization. Sorted donor p-preDC (lin−CD11c+B220+PDCA-1+) added to purified HSCs and T cells augmented GvL activity while attenuating GvHD via bidirectional signaling between donor T cells and donor pDCs with interferon gamma produced by donor T cells, inducing indoleamine 2,3-dioxygenase synthesis by donor pDCs, which limited GvHD.92 As cell sorting to enrich for small cell populations like pDCs and p-preDCs is impractical in the clinical setting, Waller et al.107 utilized CD11b selection of BM grafts to enrich for CD11b− pDC. Mice receiving mDC-depleted BM grafts had enhanced donor T-cell expansion and GvL activity versus mice receiving mDC-replete BM grafts without exacerbating GvHD. Using CD11b–depleted BM from IL-12p40KO mice, investigators showed that enhanced donor T-cell engraftment and proliferation was abrogated, demonstrating that the enhanced GvL activity seen in recipients of donor pDCs was IL-12-dependent.
Together, these pre-clinical experiments demonstrate the potential ways that pDCs and their associated type I IFN production might be exploited to attenuate GvHD and preserve, or even enhance, GvL activity. Furthermore, hematopoietic cytokines like P,44 which can increase pDC as well as modify T-cell (increase numbers of Tem and Treg) allograft content, could be used to modify subsequent risk for aGvHD in the transplant recipient while preserving GvL activity.
IMMUNE RECONSTITUTION AND INFECTION
In general, donor-derived pDC reconstitution occurs rapidly around the time of myeloid engraftment following allogeneic HCT, regardless of intensity of conditioning regimen (MA vs RIC)113 and HSC graft (BM vs PB).38 In addition, endogenous levels of FL correlate with PB myeloid and plasmacytoid DC recovery.56 In some studies, PB CD11c+CD123low (mDC) cells were noted to recover faster than PB CD11c−CD123+ (pDC)56,114,115 while other investigators did not measure a different kinetic of recovery in DC subtypes.96,97,116
CD34 and DC content within HSC graft have been studied to determine their influence on subsequent DC reconstitution in the transplant recipient (Table 3). Comparing donor CD34 and DC content between matched sibling donor G-primed PBSC and MUD BM allografts and its effect on subsequent DC reconstitution, Urbini et al.38 noted that higher CD34 dose in matched sibling donor PBSC and MUD BM grafts correlated with mDC and pDC graft content, respectively, but did not affect mDC and pDC reconstitution, respectively. In the setting of RIC, CD34+ cell dose infused with the allograft did not affect pDC recovery.117 Similarly, Clark et al.101 found no relationship between stem cell source or pDC graft content and subsequent PB pDC elevation in patients with cGvHD. In contrast to these studies, Porta et al.114 found that pDC (lin−CD11c−CD123+) graft content was higher in patients receiving PBSC (n = 11) versus BM (n = 8) allografts, and levels of circulating pDC in these patients were increased on D30 and D100 relative to BM allograft recipients.
Few studies have analyzed the function of pDC reconstituted in the blood after allogeneic HCT. Notably, Giraud et al.118 retrospectively assessed pDC function in 25 adult BMT recipients (conditioning and GvHD prophylaxis not specified, 19 with aGvHD, 10 with cGvHD, 22 were receiving immunosuppression), by counting PB CD123hiCD4− cells and measuring ex vivo HSV-1-inducible type I IFN production from peripheral blood mono-nuclear cells, and compared these with healthy donor cells. pDCs from transplant recipients were initially lower in number and reached near control levels by 14 months post transplant. In addition, transplant recipients had less HSV-1-inducible IFNα, which was not correlated to pDC. Furthermore, immunosuppressive drugs (steroids and cyclosporine inhibited inducible type I IFN when added to culture of healthy donor pDC. Together, these results suggest that susceptibility to viral infection post-allogeneic BMT may partly be due to dysfunction in reconstituted pDCs.
Like pDC function, pDC recovery and its effects on post-transplant infection risk have not been extensively studied. In 54 patients receiving RIC matched sibling donor transplant, Mohty et al.117 studied the impact of circulating levels of pDCs on transplant outcomes, including GvHD, malignant disease progression or relapse, infection and survival. The investigators noted that those patients with ‘high’ pDC recovery (⦥0.725/µL) at 3 months post transplant had improved OS in contrast to patients with a ‘low’ pDC recovery profile (< 0.725/µL) who had an increased incidence of non-relapse mortality (GvHD, infection). Furthermore, the overall incidence of late infections (viral, fungal and bacterial) was significantly higher in the ‘low’ pDC recovery group as compared with the ‘high’ pDC recovery group (59% vs 19%; P = 0.002). In multivariate analysis, only ‘high’ pDC recovery was significantly predictive of decreased death risk. Kitawaki et al.119 investigated the recovery of interferon-producing cells (lin−CD11c−CD4+CD123+) in 28 patients following MA conditioning followed by predominantly BM graft (n=17) infusion and RIC followed by PBSC graft (n =11) infusion. p-preDCs recovered to near control-level values within 30 days post transplant, but recovery was impeded by aGvHD and steroid administration. In addition, steroid administration inhibited HSV-inducible IFNα production from ex vivo peripheral blood mononuclear cells. At last, patients with lower numbers of p-preDCs had more viral infections (CMV antigenemia, adenouria, herpes zoster).
Longitudinal and cross-sectional studies of the kinetics of immune reconstitution post transplant have shown that GvHD and its associated IST are major impediments to pDC reconstitution. Numerous clinical studies have demonstrated reduced numbers of PB pDCs in both adult and pediatric transplant patients with aGvHD.96,97,116,119–122 In addition, GvHD also inhibits donor pDC development in the transplant recipient, potentially inhibiting hematopoiesis and enhancing allo-driven GvHD.90 Wikstrom and co-investigators recently have shown that mice with aGvHD are highly susceptible to murine CMV infection and fulminant CMV-associated hepatic necrosis due to impaired CMV-specific CD8+ T-cell response, resulting from GvHD-associated deficiencies in CD8+DCs, CD11b+DCs and pDCs, DC activation, and viral Ag presentation.123
Immunosuppressive therapy including steroids,118–120,124 mycophenolate mofetil,118 cyclosporine56,118 further inhibits pDC recovery and function. For example, Tajima and co-investigators co-cultured CD11b+, CD11c+ and CD11c− cells isolated from human peripheral blood mononuclear cells with cyclosporine and found that DC subtypes had reduced inducible CD80/86 expression and IFNα production.125 Chklovskaia et al.56 studied the effect of cyclosporine on ex vivo DC differentiation from CD34+ precursors and found that generated DCs had decreased ability to prime naive T cells.
SURVIVAL
pDC recovery is emerging as a key determinant of survival following allogeneic HCT (Table 3). Importantly, the kinetics of pDC recovery in transplant recipients seem to be independent of patient-, transplant- and graft-related characteristics, suggesting that post-HCT pDCs in the transplant recipient are robust biomarkers for predicting survival.122,126
CONCLUSION
pDCs assume influential roles directing immune response in the allogeneic HCT recipient (Figures 2 and 3). Whether these innate effector cells can be expanded in vivo or manipulated ex vivo to direct specific immune response in the HCT recipient is the next frontier of scientific and translational discovery to enhance their effects and ultimately to improve post-transplant outcomes.
Acknowledgments
We wish to acknowledge the many valuable contributions of investigators whose work on plasmacytoid DCs was not included given the focus of the review and space limitations.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
JJA: conception and design, primary writing of the manuscript; SMD and EKW reviewed and revised the manuscript.
REFERENCES
- 1.Pasquini MC, Wang Z. Current use and outcome of hematopoietic stem cell transplantation: CIBMTR Summary Slides. 2012 Available at http://www.cibmtr.org 2012.
- 2.Stenger EO, Turnquist HR, Mapara MY, Thomson AW. Dendritic cells and regulation of graft-versus-host disease and graft-versus-leukemia activity. Blood. 2012;119:5088–5103. doi: 10.1182/blood-2011-11-364091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rossi M, Young JW. Human dendritic cells: potent antigen-presenting cells at the crossroads of innate and adaptive immunity. J Immunol. 2005;175:1373–1381. doi: 10.4049/jimmunol.175.3.1373. [DOI] [PubMed] [Google Scholar]
- 4.Guilliams M, Ginhoux F, Jakubzick C, Naik SH, Onai N, Schraml BU, et al. Dendritic cells, monocytes and macrophages: a unified nomenclature based on ontogeny. Nat Rev Immunol. 2014;14:571–578. doi: 10.1038/nri3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cisse B, Caton ML, Lehner M, Maeda T, Scheu S, Locksley R, et al. Transcription factor E2-2 is an essential and specific regulator of plasmacytoid dendritic cell development. Cell. 2008;135:37–48. doi: 10.1016/j.cell.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maraskovsky E, Daro E, Roux E, Teepe M, Maliszewski CR, Hoek J, et al. In vivo generation of human dendritic cell subsets by Flt3 ligand. Blood. 2000;96:878–884. [PubMed] [Google Scholar]
- 7.McKenna HJ, Stocking KL, Miller RE, Brasel K, De Smedt T, Maraskovsky E, et al. Mice lacking flt3 ligand have deficient hematopoiesis affecting hematopoietic progenitor cells, dendritic cells, and natural killer cells. Blood. 2000;95:3489–3497. [PubMed] [Google Scholar]
- 8.Hacker C, Kirsch RD, Ju XS, Hieronymus T, Gust TC, Kuhl C, et al. Transcriptional profiling identifies Id2 function in dendritic cell development. Nat Immunol. 2003;4:380–386. doi: 10.1038/ni903. [DOI] [PubMed] [Google Scholar]
- 9.Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34:637–650. doi: 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 10.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 11.Kadowaki N, Ho S, Antonenko S, Malefyt RW, Kastelein RA, Bazan F, et al. Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J Exp Med. 2001;194:863–869. doi: 10.1084/jem.194.6.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mildner A, Jung S. Development and function of dendritic cell subsets. Immunity. 2014;40:642–656. doi: 10.1016/j.immuni.2014.04.016. [DOI] [PubMed] [Google Scholar]
- 13.Okamura H, Kashiwamura S, Tsutsui H, Yoshimoto T, Nakanishi K. Regulation of interferon-gamma production by IL-12 and IL-18. Curr Opin Immunol. 1998;10:259–264. doi: 10.1016/s0952-7915(98)80163-5. [DOI] [PubMed] [Google Scholar]
- 14.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 15.Walzer T, Dalod M, Robbins SH, Zitvogel L, Vivier E. Natural-killer cells and dendritic cells: “l’union fait la force”. Blood. 2005;106:2252–2258. doi: 10.1182/blood-2005-03-1154. [DOI] [PubMed] [Google Scholar]
- 16.McNab F, Mayer-Barber K, Sher A, Wack A, O’Garra A. Type I interferons in infectious disease. Nat Rev Immunol. 2015;15:87–103. doi: 10.1038/nri3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Biron CA. Activation and function of natural killer cell responses during viral infections. Curr Opin Immunol. 1997;9:24–34. doi: 10.1016/s0952-7915(97)80155-0. [DOI] [PubMed] [Google Scholar]
- 18.Matikainen S, Paananen A, Miettinen M, Kurimoto M, Timonen T, Julkunen I, et al. IFN-alpha and IL-18 synergistically enhance IFN-gamma production in human NK cells: differential regulation of Stat4 activation and IFN-gamma gene expression by IFN-alpha and IL-12. Eur J Immunol. 2001;31:2236–2245. [PubMed] [Google Scholar]
- 19.Marrack P, Kappler J, Mitchell T. Type I interferons keep activated T cells alive. J Exp Med. 1999;189:521–530. doi: 10.1084/jem.189.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun S, Zhang X, Tough DF, Sprent J. Type I interferon-mediated stimulation of T cells by CpG DNA. J Exp Med. 1998;188:2335–2342. doi: 10.1084/jem.188.12.2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gonzalez-Navajas JM, Lee J, David M, Raz E. Immunomodulatory functions of type I interferons. Nat Rev Immunol. 2012;12:125–135. doi: 10.1038/nri3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Asselin-Paturel C, Brizard G, Chemin K, Boonstra A, O’Garra A, Vicari A, et al. Type I interferon dependence of plasmacytoid dendritic cell activation and migration. J Exp Med. 2005;201:1157–1167. doi: 10.1084/jem.20041930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Porritt RA, Hertzog PJ. Dynamic control of type I IFN signalling by an integrated network of negative regulators. Trends Immunol. 2015;36:150–160. doi: 10.1016/j.it.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 24.Dzionek A, Fuchs A, Schmidt P, Cremer S, Zysk M, Miltenyi S, et al. BDCA-2, BDCA-3, and BDCA-4: three markers for distinct subsets of dendritic cells in human peripheral blood. J Immunol. 2000;165:6037–6046. doi: 10.4049/jimmunol.165.11.6037. [DOI] [PubMed] [Google Scholar]
- 25.Goubier A, Dubois B, Gheit H, Joubert G, Villard-Truc F, Asselin-Paturel C, et al. Plasmacytoid dendritic cells mediate oral tolerance. Immunity. 2008;29:464–475. doi: 10.1016/j.immuni.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Swiecki M, Colonna M. The multifaceted biology of plasmacytoid dendritic cells. Nat Rev Immunol. 2015;15:471–485. doi: 10.1038/nri3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mendelson A, Frenette PS. Hematopoietic stem cell niche maintenance during homeostasis and regeneration. Nat Med. 2014;20:833–846. doi: 10.1038/nm.3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lapidot T, Kollet O. The brain-bone-blood triad: traffic lights for stem-cell homing and mobilization. Hematology Am Soc Hematol Educ Program. 2010;2010:1–6. doi: 10.1182/asheducation-2010.1.1. [DOI] [PubMed] [Google Scholar]
- 29.Saraceni F, Shem-Tov N, Olivieri A, Nagler A. Mobilized peripheral blood grafts include more than hematopoietic stem cells: the immunological perspective. Bone Marrow Transplant. 2015;50:886–891. doi: 10.1038/bmt.2014.330. [DOI] [PubMed] [Google Scholar]
- 30.Rettig MP, Ansstas G, DiPersio JF. Mobilization of hematopoietic stem and progenitor cells using inhibitors of CXCR4 and VLA-4. Leukemia. 2012;26:34–53. doi: 10.1038/leu.2011.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fruehauf S, Tricot G. Comparison of unmobilized and mobilized graft characteristics and the implications of cell subsets on autologous and allogeneic transplantation outcomes. Biol Blood Marrow Transplant. 2010;16:1629–1648. doi: 10.1016/j.bbmt.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 32.Arpinati M, Green CL, Heimfeld S, Heuser JE, Anasetti C. Granulocyte-colony stimulating factor mobilizes T helper 2-inducing dendritic cells. Blood. 2000;95:2484–2490. [PubMed] [Google Scholar]
- 33.Arpinati M, Chirumbolo G, Urbini B, Martelli V, Stanzani M, Falcioni S, et al. Use of anti-BDCA-2 antibody for detection of dendritic cells type-2 (DC2) in allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2002;29:887–891. doi: 10.1038/sj.bmt.1703569. [DOI] [PubMed] [Google Scholar]
- 34.Morris ES, MacDonald KP, Hill GR. Stem cell mobilization with G-CSF analogs: a rational approach to separate GVHD and GVL? Blood. 2006;107:3430–3435. doi: 10.1182/blood-2005-10-4299. [DOI] [PubMed] [Google Scholar]
- 35.Daro E, Pulendran B, Brasel K, Teepe M, Pettit D, Lynch DH, et al. Polyethylene glycol-modified GM-CSF expands CD11b(high)CD11c(high) but notCD11b(low) CD11c(high) murine dendritic cells in vivo: a comparative analysis with Flt3 ligand. J Immunol. 2000;165:49–58. doi: 10.4049/jimmunol.165.1.49. [DOI] [PubMed] [Google Scholar]
- 36.Gilliet M, Boonstra A, Paturel C, Antonenko S, Xu XL, Trinchieri G, et al. The development of murine plasmacytoid dendritic cell precursors is differentially regulated by FLT3-ligand and granulocyte/macrophage colony-stimulating factor. J Exp Med. 2002;195:953–958. doi: 10.1084/jem.20020045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lonial S, Akhtari M, Kaufman J, Torre C, Lechowicz MJ, Flowers C, et al. Mobilization of hematopoietic progenitors from normal donors using the combination of granulocyte-macrophage colony-stimulating factor and granulocyte colony-stimulating factor results in fewer plasmacytoid dendritic cells in the graft and enhanced donor T cell engraftment with Th1 polarization: results from a randomized clinical trial. Biol Blood Marrow Transplant. 2013;19:460–467. doi: 10.1016/j.bbmt.2012.11.017. [DOI] [PubMed] [Google Scholar]
- 38.Urbini B, Arpinati M, Bonifazi F, Chirumbolo G, Falcioni S, Stanzani M, et al. Allogeneic graft CD34(+) cell dose correlates with dendritic cell dose and clinical outcome, but not with dendritic cell reconstitution after transplant. Exp Hematol. 2003;31:959–965. doi: 10.1016/s0301-472x(03)00232-7. [DOI] [PubMed] [Google Scholar]
- 39.Reddy V, Hill GR, Pan L, Gerbitz A, Teshima T, Brinson Y, et al. G-CSF modulates cytokine profile of dendritic cells and decreases acute graft-versus-host disease through effects on the donor rather than the recipient. Transplantation. 2000;69:691–693. doi: 10.1097/00007890-200002270-00041. [DOI] [PubMed] [Google Scholar]
- 40.Tayebi H, Kuttler F, Saas P, Lienard A, Petracca B, Lapierre V, et al. Effect of granulocyte colony-stimulating factor mobilization on phenotypical and functional properties of immune cells. Exp Hematol. 2001;29:458–470. doi: 10.1016/s0301-472x(01)00613-0. [DOI] [PubMed] [Google Scholar]
- 41.Klangsinsirikul P, Russell NH. Peripheral blood stem cell harvests from G-CSF-stimulated donors contain a skewed Th2 CD4 phenotype and a predominance of type 2 dendritic cells. Exp Hematol. 2002;30:495–501. doi: 10.1016/s0301-472x(02)00785-3. [DOI] [PubMed] [Google Scholar]
- 42.Rutella S, Filippini P, Bertaina V, Li Pira G, Altomare L, Ceccarelli S, et al. Mobilization of healthy donors with plerixafor affects the cellular composition of T-cell receptor (TCR)-alphabeta/CD19-depleted haploidentical stem cell grafts. J Transl Med. 2014;12:240. doi: 10.1186/s12967-014-0240-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kean LS, Sen S, Onabajo O, Singh K, Robertson J, Stempora L, et al. Significant mobilization of both conventional and regulatory T cells with AMD3100. Blood. 2011;118:6580–6590. doi: 10.1182/blood-2011-06-359331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Devine SM, Vij R, Rettig M, Todt L, McGlauchlen K, Fisher N, et al. Rapid mobilization of functional donor hematopoietic cells without G-CSF using AMD3100, an antagonist of the CXCR4/SDF-1 interaction. Blood. 2008;112:990–998. doi: 10.1182/blood-2007-12-130179. [DOI] [PubMed] [Google Scholar]
- 45.Zheng H, Matte-Martone C, Li H, Anderson BE, Venketesan S, Sheng Tan H, et al. Effector memory CD4+ T cells mediate graft-versus-leukemia without inducing graft-versus-host disease. Blood. 2008;111:2476–2484. doi: 10.1182/blood-2007-08-109678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rezvani K, Mielke S, Ahmadzadeh M, Kilical Y, Savani BN, Zeilah J, et al. High donor FOXP3-positive regulatory T-cell (Treg) content is associated with a low risk of GVHD following HLA-matched allogeneic SCT. Blood. 2006;108:1291–1297. doi: 10.1182/blood-2006-02-003996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miller G, Pillarisetty VG, Shah AB, Lahrs S, DeMatteo RP. Murine Flt3 ligand expands distinct dendritic cells with both tolerogenic and immunogenic properties. J Immunol. 2003;170:3554–3564. doi: 10.4049/jimmunol.170.7.3554. [DOI] [PubMed] [Google Scholar]
- 48.O’Keeffe M, Hochrein H, Vremec D, Pooley J, Evans R, Woulfe S, et al. Effects of administration of progenipoietin 1, Flt-3 ligand, granulocyte colony-stimulating factor, and pegylated granulocyte-macrophage colony-stimulating factor on dendritic cell subsets in mice. Blood. 2002;99:2122–2130. doi: 10.1182/blood.v99.6.2122. [DOI] [PubMed] [Google Scholar]
- 49.Anandasabapathy N, Breton G, Hurley A, Caskey M, Trumpfheller C, Sarma P, et al. Efficacy and safety of CDX-301, recombinant human Flt3L, at expanding dendritic cells and hematopoietic stem cells in healthy human volunteers. Bone Marrow Transplant. 2015;50:924–930. doi: 10.1038/bmt.2015.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Teshima T, Reddy P, Lowler KP, KuKuruga MA, Liu C, Cooke KR, et al. Flt3 ligand therapy for recipients of allogeneic bone marrow transplants expands host CD8 alpha(+) dendritic cells and reduces experimental acute graft-versus-host disease. Blood. 2002;99:1825–1832. doi: 10.1182/blood.v99.5.1825. [DOI] [PubMed] [Google Scholar]
- 51.Blazar BR, McKenna HJ, Panoskaltsis-Mortari A, Taylor PA. Flt3 ligand (FL) treatment of murine donors does not modify graft-versus-host disease (GVHD) but FL treatment of recipients post-bone marrow transplantation accelerates GVHD lethality. Biol Blood Marrow Transplant. 2001;7:197–207. doi: 10.1053/bbmt.2001.v7.pm11349806. [DOI] [PubMed] [Google Scholar]
- 52.Fry TJ, Sinha M, Milliron M, Chu YW, Kapoor V, Gress RE, et al. Flt3 ligand enhances thymic-dependent and thymic-independent immune reconstitution. Blood. 2004;104:2794–2800. doi: 10.1182/blood-2003-11-3789. [DOI] [PubMed] [Google Scholar]
- 53.Wils EJ, Braakman E, Verjans GM, Rombouts EJ, Broers AE, Niesters HG, et al. Flt3 ligand expands lymphoid progenitors prior to recovery of thymopoiesis and accelerates T cell reconstitution after bone marrow transplantation. J Immunol. 2007;178:3551–3557. doi: 10.4049/jimmunol.178.6.3551. [DOI] [PubMed] [Google Scholar]
- 54.Yunusov MY, Georges GE, Storb R, Moore P, Hagglund H, Affolter V, et al. FLT3 ligand promotes engraftment of allogeneic hematopoietic stem cells without significant graft-versus-host disease. Transplantation. 2003;75:933–940. doi: 10.1097/01.TP.0000057831.93385.7D. [DOI] [PubMed] [Google Scholar]
- 55.He S, Chu J, Vasu S, Deng Y, Yuan S, Zhang J, et al. FLT3L and plerixafor combination increases hematopoietic stem cell mobilization and leads to improved transplantation outcome. Biol Blood Marrow Transplant. 2014;20:309–313. doi: 10.1016/j.bbmt.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chklovskaia E, Nowbakht P, Nissen C, Gratwohl A, Bargetzi M, Wodnar-Filipowicz A. Reconstitution of dendritic and natural killer-cell subsets after allogeneic stem cell transplantation: effects of endogenous flt3 ligand. Blood. 2004;103:3860–3868. doi: 10.1182/blood-2003-04-1200. [DOI] [PubMed] [Google Scholar]
- 57.Bjorck P. Isolation and characterization of plasmacytoid dendritic cells from Flt3 ligand and granulocyte-macrophage colony-stimulating factor-treated mice. Blood. 2001;98:3520–3526. doi: 10.1182/blood.v98.13.3520. [DOI] [PubMed] [Google Scholar]
- 58.Pulendran B, Banchereau J, Burkeholder S, Kraus E, Guinet E, Chalouni C, et al. Flt3-ligand and granulocyte colony-stimulating factor mobilize distinct human dendritic cell subsets in vivo. J Immunol. 2000;165:566–572. doi: 10.4049/jimmunol.165.1.566. [DOI] [PubMed] [Google Scholar]
- 59.Mielcarek M, Graf L, Johnson G, Torok-Storb B. Production of interleukin-10 by granulocyte colony-stimulating factor-mobilized blood products: a mechanism for monocyte-mediated suppression of T-cell proliferation. Blood. 1998;92:215–222. [PubMed] [Google Scholar]
- 60.Rutella S, Bonanno G, Pierelli L, Mariotti A, Capoluongo E, Contemi AM, et al. Granulocyte colony-stimulating factor promotes the generation of regulatory DC through induction of IL-10 and IFN-alpha. Eur J Immunol. 2004;34:1291–1302. doi: 10.1002/eji.200324651. [DOI] [PubMed] [Google Scholar]
- 61.Lundqvist A, Smith AL, Takahashi Y, Wong S, Bahceci E, Cook L, et al. Differences in the phenotype, cytokine gene expression profiles, and in vivo alloreactivity of T cells mobilized with plerixafor compared with G-CSF. J Immunol. 2013;191:6241–6249. doi: 10.4049/jimmunol.1301148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Heazlewood SY, Oteiza A, Cao H, Nilsson SK. Analyzing hematopoietic stem cell homing, lodgment, and engraftment to better understand the bone marrow niche. Ann N Y Acad Sci. 2014;1310:119–128. doi: 10.1111/nyas.12329. [DOI] [PubMed] [Google Scholar]
- 63.Hanash AM, Levy RB. Donor CD4+CD25+ T cells promote engraftment and tolerance following MHC-mismatched hematopoietic cell transplantation. Blood. 2005;105:1828–1836. doi: 10.1182/blood-2004-08-3213. [DOI] [PubMed] [Google Scholar]
- 64.Gandy KL, Domen J, Aguila H, Weissman IL. CD8+TCR+ and CD8+TCR- cells in whole bone marrow facilitate the engraftment of hematopoietic stem cells across allogeneic barriers. Immunity. 1999;11:579–590. doi: 10.1016/s1074-7613(00)80133-8. [DOI] [PubMed] [Google Scholar]
- 65.Kaufman CL, Colson YL, Wren SM, Watkins S, Simmons RL, Ildstad ST. Phenotypic characterization of a novel bone marrow-derived cell that facilitates engraftment of allogeneic bone marrow stem cells. Blood. 1994;84:2436–2446. [PubMed] [Google Scholar]
- 66.Fugier-Vivier IJ, Rezzoug F, Huang Y, Graul-Layman AJ, Schanie CL, Xu H, et al. Plasmacytoid precursor dendritic cells facilitate allogeneic hematopoietic stem cell engraftment. J Exp Med. 2005;201:373–383. doi: 10.1084/jem.20041399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Huang Y, Bozulic LD, Miller T, Xu H, Hussain LR, Ildstad ST. CD8α+ plasmacytoid precursor DCs induce antigen-specific regulatory T cells that enhance HSC engraftment in vivo. Blood. 2011;117:2494–2505. doi: 10.1182/blood-2010-06-291187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Taylor KN, Shinde-Patil VR, Cohick E, Colson YL. Induction of FoxP3+CD4+25+ regulatory T cells following hemopoietic stem cell transplantation: role of bone marrow-derived facilitating cells. J Immunol. 2007;179:2153–2162. doi: 10.4049/jimmunol.179.4.2153. [DOI] [PubMed] [Google Scholar]
- 69.Colson YL, Christopher K, Glickman J, Taylor KN, Wright R, Perkins DL. Absence of clinical GVHD and the in vivo induction of regulatory T cells after transplantation of facilitating cells. Blood. 2004;104:3829–3835. doi: 10.1182/blood-2004-01-0393. [DOI] [PubMed] [Google Scholar]
- 70.Rezzoug F, Huang Y, Tanner MK, Wysoczynski M, Schanie CL, Chilton PM, et al. TNF-alpha is critical to facilitate hemopoietic stem cell engraftment and function. J Immunol. 2008;180:49–57. doi: 10.4049/jimmunol.180.1.49. [DOI] [PubMed] [Google Scholar]
- 71.Retting MPMK, Ritchey J, Holt M, Deych E, Lopeaz S, Gabriel J, et al. Preferential mobilization of CD34+ plasmacytoid dendirtic cell precursors by plerixafor. Blood (ASH Annual Meeting Abstracts) 2009:114. abstract 32. [Google Scholar]
- 72.Larochelle A, Krouse A, Metzger M, Orlic D, Donahue RE, Fricker S, et al. AMD3100 mobilizes hematopoietic stem cells with long-term repopulating capacity in nonhuman primates. Blood. 2006;107:3772–3778. doi: 10.1182/blood-2005-09-3592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fruehauf S, Veldwijk MR, Seeger T, Schubert M, Laufs S, Topaly J, et al. A combination of granulocyte-colony-stimulating factor (G-CSF) and plerixafor mobilizes more primitive peripheral blood progenitor cells than G-CSF alone: results of a European phase II study. Cytotherapy. 2009;11:992–1001. doi: 10.3109/14653240903121245. [DOI] [PubMed] [Google Scholar]
- 74.Shlomchik WD, Couzens MS, Tang CB, McNiff J, Robert ME, Liu J, et al. Prevention of graft versus host disease by inactivation of host antigen-presenting cells. Science. 1999;285:412–415. doi: 10.1126/science.285.5426.412. [DOI] [PubMed] [Google Scholar]
- 75.Duffner UA, Maeda Y, Cooke KR, Reddy P, Ordemann R, Liu C, et al. Host dendritic cells alone are sufficient to initiate acute graft-versus-host disease. J Immunol. 2004;172:7393–7398. doi: 10.4049/jimmunol.172.12.7393. [DOI] [PubMed] [Google Scholar]
- 76.Markey KA, Banovic T, Kuns RD, Olver SD, Don AL, Raffelt NC, et al. Conventional dendritic cells are the critical donor APC presenting alloantigen after experimental BMT. Blood. 2009;113:5644–5649. doi: 10.1182/blood-2008-12-191833. [DOI] [PubMed] [Google Scholar]
- 77.Koyama M, Hashimoto D, Aoyama K, Matsuoka K, Karube K, Niiro H, et al. Plasmacytoid dendritic cells prime alloreactive T cells to mediate graft-versus-host disease as antigen-presenting cells. Blood. 2009;113:2088–2095. doi: 10.1182/blood-2008-07-168609. [DOI] [PubMed] [Google Scholar]
- 78.Li H, Demetris AJ, McNiff J, Matte-Martone C, Tan HS, Rothstein DM, et al. Profound depletion of host conventional dendritic cells, plasmacytoid dendritic cells, and B cells does not prevent graft-versus-host disease induction. J Immunol. 2012;188:3804–3811. doi: 10.4049/jimmunol.1102795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bossard C, Malard F, Arbez J, Chevallier P, Guillaume T, Delaunay J, et al. Plasmacytoid dendritic cells and Th17 immune response contribution in gastrointestinal acute graft-versus-host disease. Leukemia. 2012;26:1471–1474. doi: 10.1038/leu.2012.41. [DOI] [PubMed] [Google Scholar]
- 80.Yu CF, Peng WM, Oldenburg J, Hoch J, Bieber T, Limmer A, et al. Human plasmacytoid dendritic cells support Th17 cell effector function in response to TLR7 ligation. J Immunol. 2010;184:1159–1167. doi: 10.4049/jimmunol.0901706. [DOI] [PubMed] [Google Scholar]
- 81.Isaksson M, Ardesjo B, Ronnblom L, Kampe O, Lassmann H, Eloranta ML, et al. Plasmacytoid DC promote priming of autoimmune Th17 cells and EAE. Eur J Immunol. 2009;39:2925–2935. doi: 10.1002/eji.200839179. [DOI] [PubMed] [Google Scholar]
- 82.Wendland M, Czeloth N, Mach N, Malissen B, Kremmer E, Pabst O, et al. CCR9 is a homing receptor for plasmacytoid dendritic cells to the small intestine. Proc Natl Acad Sci USA. 2007;104:6347–6352. doi: 10.1073/pnas.0609180104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hadeiba H, Sato T, Habtezion A, Oderup C, Pan J, Butcher EC. CCR9 expression defines tolerogenic plasmacytoid dendritic cells able to suppress acute graft-versus-host disease. Nat Immunol. 2008;9:1253–1260. doi: 10.1038/ni.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wakkach A, Fournier N, Brun V, Breittmayer JP, Cottrez F, Groux H. Characterization of dendritic cells that induce tolerance and T regulatory 1 cell differentiation in vivo. Immunity. 2003;18:605–617. doi: 10.1016/s1074-7613(03)00113-4. [DOI] [PubMed] [Google Scholar]
- 85.Hanabuchi S, Ito T, Park WR, Watanabe N, Shaw JL, Roman E, et al. Thymic stromal lymphopoietin-activated plasmacytoid dendritic cells induce the generation of FOXP3+ regulatory T cells in human thymus. J Immunol. 2010;184:2999–3007. doi: 10.4049/jimmunol.0804106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Moseman EA, Liang X, Dawson AJ, Panoskaltsis-Mortari A, Krieg AM, Liu YJ, et al. Human plasmacytoid dendritic cells activated by CpG oligodeoxynucleotides induce the generation of CD4+CD25+ regulatory T cells. J Immunol. 2004;173:4433–4442. doi: 10.4049/jimmunol.173.7.4433. [DOI] [PubMed] [Google Scholar]
- 87.Gilliet M, Liu YJ. Human plasmacytoid-derived dendritic cells and the induction of T-regulatory cells. Hum Immunol. 2002;63:1149–1155. doi: 10.1016/s0198-8859(02)00753-x. [DOI] [PubMed] [Google Scholar]
- 88.Sela U, Olds P, Park A, Schlesinger SJ, Steinman RM. Dendritic cells induce antigen-specific regulatory T cells that prevent graft versus host disease and persist in mice. J Exp Med. 2011;208:2489–2496. doi: 10.1084/jem.20110466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Trzonkowski P, Dukat-Mazurek A, Bieniaszewska M, Marek-Trzonkowska N, Dobyszuk A, Juscinska J, et al. Treatment of graft-versus-host disease with naturally occurring T regulatory cells. BioDrugs. 2013;27:605–614. doi: 10.1007/s40259-013-0050-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Banovic T, Markey KA, Kuns RD, Olver SD, Raffelt NC, Don AL, et al. Graft-versus-host disease prevents the maturation of plasmacytoid dendritic cells. J Immunol. 2009;182:912–920. doi: 10.4049/jimmunol.182.2.912. [DOI] [PubMed] [Google Scholar]
- 91.Li JM, Southerland LT, Lu Y, Darlak KA, Giver CR, McMillin DW, et al. Activation, immune polarization, and graft-versus-leukemia activity of donor T cells are regulated by specific subsets of donor bone marrow antigen-presenting cells in allogeneic hemopoietic stem cell transplantation. J Immunol. 2009;183:7799–7809. doi: 10.4049/jimmunol.0900155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lu Y, Giver CR, Sharma A, Li JM, Darlak KA, Owens LM, et al. IFN-gamma and indoleamine 2,3-dioxygenase signaling between donor dendritic cells and T cells regulates graft versus host and graft versus leukemia activity. Blood. 2012;119:1075–1085. doi: 10.1182/blood-2010-12-322891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Waller EK, Rosenthal H, Sagar L. DC2 effect on survival following allogeneic bone marrow transplantation. Oncology (Williston Park) 2002;16(1 Suppl 1):19–26. [PubMed] [Google Scholar]
- 94.Waller EK, Rosenthal H, Jones TW, Peel J, Lonial S, Langston A, et al. Larger numbers of CD4(bright) dendritic cells in donor bone marrow are associated with increased relapse after allogeneic bone marrow transplantation. Blood. 2001;97:2948–2956. doi: 10.1182/blood.v97.10.2948. [DOI] [PubMed] [Google Scholar]
- 95.Waller EK, Logan BR, Harris WA, Devine SM, Porter DL, Mineishi S, et al. Improved survival after transplantation of more donor plasmacytoid dendritic or naive T cells from unrelated-donor marrow grafts: results from BMTCTN 0201. J Clin Oncol. 2014;32:2365–2372. doi: 10.1200/JCO.2013.54.4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Horvath R, Budinsky V, Kayserova J, Kalina T, Formankova R, Stary J, et al. Kinetics of dendritic cells reconstitution and costimulatory molecules expression after myeloablative allogeneic haematopoetic stem cell transplantation: implications for the development of acute graft-versus host disease. Clin Immunol. 2009;131:60–69. doi: 10.1016/j.clim.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 97.Vakkila J, Thomson AW, Hovi L, Vettenranta K, Saarinen-Pihkala UM. Circulating dendritic cell subset levels after allogeneic stem cell transplantation in children correlate with time post transplant and severity of acute graft-versus-host disease. Bone Marrow Transplant. 2005;35:501–507. doi: 10.1038/sj.bmt.1704827. [DOI] [PubMed] [Google Scholar]
- 98.Rajasekar R, Lakshmi KM, George B, Viswabandya A, Thirugnanam R, Abraham A, et al. Dendritic cell count in the graft predicts relapse in patients with hema-tologic malignancies undergoing an HLA-matched related allogeneic peripheral blood stem cell transplant. Biol Blood Marrow Transplant. 2010;16:854–860. doi: 10.1016/j.bbmt.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 99.Pihusch M, Boeck S, Hamann M, Pihusch V, Heller T, Diem H, et al. Peripheral dendritic cell chimerism in allogeneic hematopoietic stem cell recipients. Transplantation. 2005;80:843–849. doi: 10.1097/01.tp.0000174339.17567.a0. [DOI] [PubMed] [Google Scholar]
- 100.Chan GW, Gorgun G, Miller KB, Foss FM. Persistence of host dendritic cells after transplantation is associated with graft-versus-host disease. Biol Blood Marrow Transplant. 2003;9:170–176. doi: 10.1053/bbmt.2003.50006. [DOI] [PubMed] [Google Scholar]
- 101.Clark FJ, Freeman L, Dzionek A, Schmitz J, McMullan D, Simpson P, et al. Origin and subset distribution of peripheral blood dendritic cells in patients with chronic graft-versus-host disease. Transplantation. 2003;75:221–225. doi: 10.1097/01.TP.0000041783.34083.11. [DOI] [PubMed] [Google Scholar]
- 102.MacDonald KP, Rowe V, Filippich C, Thomas R, Clouston AD, Welply JK, et al. Donor pretreatment with progenipoietin-1 is superior to granulocyte colony-stimulating factor in preventing graft-versus-host disease after allogeneic stem cell transplantation. Blood. 2003;101:2033–2042. doi: 10.1182/blood-2002-05-1529. [DOI] [PubMed] [Google Scholar]
- 103.MacDonald KP, Rowe V, Clouston AD, Welply JK, Kuns RD, Ferrara JL, et al. Cytokine expanded myeloid precursors function as regulatory antigen-presenting cells and promote tolerance through IL-10-producing regulatory T cells. J Immunol. 2005;174:1841–1850. doi: 10.4049/jimmunol.174.4.1841. [DOI] [PubMed] [Google Scholar]
- 104.Toubai T, Mathewson N, Reddy P. The role of dendritic cells in graft-versus-tumor effect. Front Immunol. 2014;5:66. doi: 10.3389/fimmu.2014.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Belardelli F, Ferrantini M, Proietti E, Kirkwood JM. Interferon-alpha in tumor immunity and immunotherapy. Cytokine Growth Factor Rev. 2002;13:119–134. doi: 10.1016/s1359-6101(01)00022-3. [DOI] [PubMed] [Google Scholar]
- 106.Lonial S, Torre C, David E, Harris W, Arellano M, Waller EK. Regulation of alloimmune responses by dendritic cell subsets. Exp Hematol. 2008;36:1309–1317. doi: 10.1016/j.exphem.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 107.Darlak KA, Wang Y, Li JM, Harris WA, Owens LM, Waller EK. Enrichment of IL-12-producing plasmacytoid dendritic cells in donor bone marrow grafts enhances graft-versus-leukemia activity in allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2013;19:1331–1339. doi: 10.1016/j.bbmt.2013.06.016. [DOI] [PubMed] [Google Scholar]
- 108.Borges L, Miller RE, Jones J, Ariail K, Whitmore J, Fanslow W, et al. Synergistic action of fms-like tyrosine kinase 3 ligand and CD40 ligand in the induction of dendritic cells and generation of antitumor immunity in vivo. J Immunol. 1999;163:1289–1297. [PubMed] [Google Scholar]
- 109.Robb RJ, Kreijveld E, Kuns RD, Wilson YA, Olver SD, Don AL, et al. Type I-IFNs control GVHD and GVL responses after transplantation. Blood. 2011;118:3399–3409. doi: 10.1182/blood-2010-12-325746. [DOI] [PubMed] [Google Scholar]
- 110.Malmgaard L, Salazar-Mather TP, Lewis CA, Biron CA. Promotion of alpha/beta interferon induction during in vivo viral infection through alpha/beta interferon receptor/STAT1 system-dependent and -independent pathways. J Virol. 2002;76:4520–4525. doi: 10.1128/JVI.76.9.4520-4525.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Capitini CM, Nasholm NM, Chien CD, Larabee SM, Qin H, Song YK, et al. Absence of STAT1 in donor-derived plasmacytoid dendritic cells results in increased STAT3 and attenuates murine GVHD. Blood. 2014;124:1976–1986. doi: 10.1182/blood-2013-05-500876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Li JM, Waller EK. Donor antigen-presenting cells regulate T-cell expansion and antitumor activity after allogeneic bone marrow transplantation. Biol Blood Marrow Transplant. 2004;10:540–551. doi: 10.1016/j.bbmt.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 113.Auffermann-Gretzinger S, Lossos IS, Vayntrub TA, Leong W, Grumet FC, Blume KG, et al. Rapid establishment of dendritic cell chimerism in allogeneic hematopoietic cell transplant recipients. Blood. 2002;99:1442–1448. doi: 10.1182/blood.v99.4.1442. [DOI] [PubMed] [Google Scholar]
- 114.Porta MD, Rigolin GM, Alessandrino EP, Maiocchi M, Malcovati L, Vanelli L, et al. Dendritic cell recovery after allogeneic stem-cell transplantation in acute leukemia: correlations with clinical and transplant characteristics. Eur J Haematol. 2004;72:18–25. doi: 10.1046/j.0902-4441.2004.00172.x. [DOI] [PubMed] [Google Scholar]
- 115.Morse MA, Rizzieri D, Stenzel TT, Hobeika AC, Vredenburgh JJ, Chao NJ, et al. Dendritic cell recovery following nonmyeloablative allogeneic stem cell transplants. J Hematother Stem Cell Res. 2002;11:659–668. doi: 10.1089/15258160260194802. [DOI] [PubMed] [Google Scholar]
- 116.Fagnoni FF, Oliviero B, Giorgiani G, De Stefano P, Deho A, Zibera C, et al. Reconstitution dynamics of plasmacytoid and myeloid dendritic cell precursors after allogeneic myeloablative hematopoietic stem cell transplantation. Blood. 2004;104:281–289. doi: 10.1182/blood-2003-07-2443. [DOI] [PubMed] [Google Scholar]
- 117.Mohty M, Blaise D, Faucher C, Bardou VJ, Gastaut JA, Viens P, et al. Impact of plasmacytoid dendritic cells on outcome after reduced-intensity conditioning allogeneic stem cell transplantation. Leukemia. 2005;19:1–6. doi: 10.1038/sj.leu.2403558. [DOI] [PubMed] [Google Scholar]
- 118.Giraud S, Dhedin N, Gary-Gouy H, Lebon P, Vernant JP, Dalloul A. Plasmacytoid dendritic cell reconstitution following bone marrow transplantation: subnormal recovery and functional deficit of IFN-alpha/beta production in response to herpes simplex virus. J Interferon Cytokine Res. 2005;25:135–143. doi: 10.1089/jir.2005.25.135. [DOI] [PubMed] [Google Scholar]
- 119.Kitawaki T, Kadowaki N, Ishikawa T, Ichinohe T, Uchiyama T. Compromised recovery of natural interferon-alpha/beta-producing cells after allogeneic hematopoietic stem cell transplantation complicated by acute graft-versus-host disease and glucocorticoid administration. Bone Marrow Transplant. 2003;32:187–194. doi: 10.1038/sj.bmt.1704093. [DOI] [PubMed] [Google Scholar]
- 120.Arpinati M, Chirumbolo G, Urbini B, Bonifazi F, Bandini G, Saunthararajah Y, et al. Acute graft-versus-host disease and steroid treatment impair CD11c+ and CD123+ dendritic cell reconstitution after allogeneic peripheral blood stem cell transplantation. Biol Blood Marrow Transplant. 2004;10:106–115. doi: 10.1016/j.bbmt.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 121.Watanabe N, Narita M, Furukawa T, Nakamura T, Yamahira A, Masuko M, et al. Kinetics of pDCs, mDCs, gammadeltaT cells and regulatory T cells in association with graft versus host disease after hematopoietic stem cell transplantation. Int J Lab Hematol. 2011;33:378–390. doi: 10.1111/j.1751-553X.2011.01300.x. [DOI] [PubMed] [Google Scholar]
- 122.Peric Z, Cahu X, Malard F, Brissot E, Chevallier P, Guillaume T, et al. Peripheral blood plasmacytoid dendritic cells at day 100 can predict outcome after allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2015;21:1431–1436. doi: 10.1016/j.bbmt.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 123.Wikstrom ME, Fleming P, Kuns RD, Schuster IS, Voigt V, Miller G, et al. Acute graft-versus-host disease results in a severe DC defect that prevents T cell priming and leads to fulminant cytomegalovirus disease in mice. Blood. 2015;126:1503–1514. doi: 10.1182/blood-2015-01-622837. [DOI] [PubMed] [Google Scholar]
- 124.Boor PP, Metselaar HJ, Mancham S, Tilanus HW, Kusters JG, Kwekkeboom J. Prednisolone suppresses the function and promotes apoptosis of plasmacytoid dendritic cells. Am J Transplant. 2006;6:2332–2341. doi: 10.1111/j.1600-6143.2006.01476.x. [DOI] [PubMed] [Google Scholar]
- 125.Tajima K, Amakawa R, Ito T, Miyaji M, Takebayashi M, Fukuhara S. Immunomodulatory effects of cyclosporin A on human peripheral blood dendritic cell subsets. Immunology. 2003;108:321–328. doi: 10.1046/j.1365-2567.2003.01585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Goncalves MV, Yamamoto M, Kimura EY, Rensi Colturato VA, Pedro de Souza M, Mauad M, et al. Lowcounts of plasmacytoid dendritic cells after engraftment are associated with high early mortality after allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2015;21:1223–1229. doi: 10.1016/j.bbmt.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 127.Leventhal J, Abecassis M, Miller J, Gallon L, Ravindra K, Tollerud DJ, et al. Chimerism and tolerance without GVHD or engraftment syndrome in HLA-mismatched combined kidney and hematopoietic stem cell transplantation. Sci Transl Med. 2012;4 doi: 10.1126/scitranslmed.3003509. 124ra28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Arbez J, Saas P, Lamarthee B, Malard F, Couturier M, Mohty M, et al. Impact of donor hematopoietic cells mobilized with G-CSF and plerixafor on murine acute graft-versus-host-disease. Cytotherapy. 2015;17:948–955. doi: 10.1016/j.jcyt.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 129.Pulendran B, Smith JL, Caspary G, Brasel K, Pettit D, Maraskovsky E, et al. Distinct dendritic cell subsets differentially regulate the class of immune response in vivo. Proc Natl Acad Sci USA. 1999;96:1036–1041. doi: 10.1073/pnas.96.3.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bertaina A, Merli P, Rutella S, Pagliara D, Bernardo ME, Masetti R, et al. HLA-haploidentical stem cell transplantation after removal of alphabeta+ T and B cells in children with nonmalignant disorders. Blood. 2014;124:822–826. doi: 10.1182/blood-2014-03-563817. [DOI] [PubMed] [Google Scholar]
- 131.Reddy V, Iturraspe JA, Tzolas AC, Meier-Kriesche HU, Schold J, Wingard JR. Low dendritic cell count after allogeneic hematopoietic stem cell transplantation predicts relapse, death, and acute graft-versus-host disease. Blood. 2004;103:4330–4335. doi: 10.1182/blood-2003-09-3325. [DOI] [PubMed] [Google Scholar]
- 132.Rajasekar R, Mathews V, Lakshmi KM, Sellathamby S, George B, Viswabandya A, et al. Plasmacytoid dendritic cell count on day 28 in HLA-matched related allogeneic peripheral blood stem cell transplant predicts the incidence of acute and chronic GVHD. Biol Blood Marrow Transplant. 2008;14:344–350. doi: 10.1016/j.bbmt.2007.12.494. [DOI] [PubMed] [Google Scholar]
- 133.Abe M, Thomson AW. Dexamethasone preferentially suppresses plasmacytoid dendritic cell differentiation and enhances their apoptotic death. Clin Immunol. 2006;118:300–306. doi: 10.1016/j.clim.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 134.Talarn C, Urbano-Ispizua A, Martino R, Perez-Simon JA, Batlle M, Herrera C, et al. Kinetics of recovery of dendritic cell subsets after reduced-intensity conditioning allogeneic stem cell transplantation and clinical outcome. Haematologica. 2007;92:1655–1663. doi: 10.3324/haematol.11076. [DOI] [PubMed] [Google Scholar]