Abstract
Background
The objective of this study was to investigate the effects of maternal high fat intake on intestinal development and transcriptional profile.
Methods
Eight gilts with similar age and body weight were randomly allocated into 2 groups receiving the control and high fat diets (HF diet) from d 30 to 90 of gestation, with 4 gilts each group and one gilt each pen. At d 90 of gestation, two fetuses each gilt were removed by cesarean section. Intestinal samples were collected for analysis of morphology, enzyme activities and transcriptional profile.
Results
The results showed that feeding HF diet markedly increased the fetal weight and lactase activity, also tended to increase intestinal morphology. Porcine Oligo Microarray analysis indicated that feeding HF diet inhibited 64 % of genes (39 genes down-regulated while 22 genes up-regulated),which were related to immune response, cancer and metabolism, also markedly modified 33 signal pathways such as antigen processing and presentation, intestinal immune network for IgA production, Jak-STAT and TGF-ß signaling transductions, pathways in colorectal cancer and glycerolipid metabolism.
Conclusion
Collectively, it could be concluded that maternal high fat intake was able to increase fetal weight and lactase activity, however, it altered the intestinal immune response, signal transduction and metabolism.
Electronic supplementary material
The online version of this article (doi:10.1186/s12944-016-0261-0) contains supplementary material, which is available to authorized users.
Keywords: Maternal nutrition, Offspring, Immune, Cancer, DNA microarray
Background
Gastrointestinal tract (GIT), as an internal organ to digest nutrients and resist exogenous antigens, starts to develop at early gestation and mature rapidly in late gestation for extra-uterine life [1]. The functional maturation of GIT occurs in both pre- and postnatal period, which is largely influenced by maternal nutrition [2]. Maternal diet has been shown to affect the fetal development and organ function in mammalian animals [3]. Our recent study also suggests that maternal nutrition levels could affect the intestinal development and function, in which maternal over-nutrition would improve intestinal morphology, enzyme activities and gene expressions of nutrient transporters in newborn pigs [4]. However, it has been reported that maternal high-fat intake or –related obesity could impair gut barrier, enhance gene expression of pro-inflammatory cytokines in offspring intestine, thus predisposes offspring to inflammatory bowel disease [5, 6]. However, the underlying mechanism for the effects of maternal high fat intake on the intestinal development and function are limited. The current study was designed to investigate the effects of maternal high fat intake on fetal intestinal development and function by measuring parameters on morphology, enzyme activities and transcriptional profiles. Oligo Microarray was used to analyze the genomic response of fetal intestine to maternal high fat intake. Pigs were chosen as the experimental animal, because it is generally accepted to be closer to humans than other laboratory or domestic animals in terms of gastrointestinal anatomy, physiology, nutrition and microbiota [2, 7–9].
Methods
The experimental procedure was approved by the University of Sichuan Agricultural Animal Care Advisory committee, and followed the current law of animal protection.
Animals and diets
A total of 8 Meishan (MS) gilts (aged at 266 ± 15 d, initial body weight at 73 ± 4 kg) were used in this study. After inseminated with MS semen, eight gilts were randomly allocated to receive control diet (CON diet with 14 % Protein, 34.7 % Starch and 2.8 % Fat) and high fat diet (HF diet with 14 % Protein, 34.7 % Starch and 7.3 % Fat), respectively. The 4.5 % of soy oil was added into CON diet to formulate HF diet, as a result, HF diet contained digestive energy (DE) at 3.0 Mcal/kg, while CON diet contained DE at 2.6 Mcal/kg. According to the fatty acids contents of feed ingredients by NRC (2012), the contents of saturated, mono- and polyunsaturated fatty acids were 0.25 %, 0.48 %, 0.83 % in CON diet and 0.84 %, 1.78 %, 3.44 % in HF diet, respectively. The other nutrient levels were similar between 2 diets, meeting or exceeding nutrient requirements recommended by NRC (2012). All gilts were housed individually in stall (2.5 m length × 1.6 m width), receiving the same amount of diets at 2.0 kg from d 1 to 30 of gestation and 2.5 kg from d 30 to 90 of gestation, with free access to water. Environmental temperature was maintained at approximately 24 °C during the experiment.
Sample collection
At d 90 of gestation, gilts were weighed (in average 128 kg at HF vs. 117 kg at CON group) and anaesthetized by intramuscularly injecting Zoletil 50 at the dose of 0.1 mg/kg (Virbac, France), then the uterus were removed from gilts. Two fetuses near the average fetal weight were collected each gilt. As the previous study, duodenal, jejunal and ileal samples (approximately 2 cm) were preserved in 4 % paraformaldehyde solution, then embedded in paraffin. Each tissue sample of duodenum, jejunum and ileum was used to prepare 5 slides, each slide had three sections (5 mm thickness), which were stained with eosin and haematoxylin, 20 well-oriented villi and crypts each section were measured for morphology (Optimus software version 6.5, Media Cybergenetics, North Reading, MA, USA), and villous height to crypt depth ratio (VCR) was calculated [10]. A section of duodenum, jejunum and ileum tissues were collected and snap-frozen in liquid nitrogen, then stored at −80 °C for analysis of enzyme activities, RNA microarray and gene expression.
Enzyme activities
According to the previous study, the thawing samples of jejunum and ileum were weighed (approximately 2 g), then 9 times volume of 50 mM Tris–HCl buffer (pH 7 · 0) than the sample weight were added and homogenized for 40 s by homogenate machine (Homogenizer Power Gen 125™, ThermoFisher Scientific, MA, USA) and centrifuged at 3000 g for 10 min, the supernatant was collected and stored at −20 °C [11]. Total protein was extracted from the supernatant and protein concentration was determined by bicinchoninic acid protein assay with bovine serum albumin as the standard (Solarbio, Inc., Beijing, China). Activities of disaccharidase including maltase, sucrase and lactase were measured using commercial kits (Nanjing Jiancheng Bioengineering, Nanjing, China). The absorbance at 450 nm was determined with spectrophotometer (Beckman Coulter DU-800; Beckman Coulter, Inc., CA, USA). Activities of disaccharidase were presented as U/mg protein. One unit (U) was defined by 1 nmol of maltose, sucrose and lactose as a substrate for the enzymatic reaction, respectively.
RNA extraction
The frozen ileum tissues were used for RNA extraction, 4 sections around luminal circle each tissue were collected and pooled for RNA extraction. Total RNA was extracted using Trizol reagent (Invitrogen, Carlsbad, CA, USA) and quantified using spectrophotometry based on absorbance at 260 nm, the RNA quality was monitored using Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA, USA). The equal amount of RNA from 2 fetus each gilt were pooled together.
Porcine oligo microarray
As in our previous study, Agilent Porcine Oligo Microarray (4 × 44 K) containing more than 40,000 probes were used [12]. Cyanine-3 (Cy3)-labeled cRNA was prepared from 0.5 μg RNA using the One-Color Low RNA Input Linear Amplification PLUS kit (Agilent Technologies,Palo Alto, CA, USA) according to the manufacturer’s instructions, and followed by the RNeasy column purification (Qiagen, Valencia, CA, USA). Dye incorporation and cRNA yield were checked with the NanoDrop ND-1000 Spectrophotometer. Microarrays were hybridized at 65 °C for 17 h and washed with a Gene Expression Washing Buffer Kit (Agilent Technologies, Palo Alto, CA, USA). Slides were scanned with an Agilent microarray scanner.
Microarray data collection and analysis
Microarray data were collected and analyzed using Agilent G2567AA Feature Extraction software, following Agilent’s direct labeling protocol. The quantile method was used to normalize the probe intensities across the whole set of arrays. Three criteria were used to determine statistically significant differential expression of intestinal genes between fetus from CON and HF gilts: 1) statistical significance: P value as determined by t-test < 0.05; 2) reliability: a spot quality flag P (“P,” a quality flag assigned by the software package); 3) relevance: a minimal fold change between the means of the 2 groups >1.5.
Real-time PCR
In order to verify the microarray data, RNA samples used for porcine oligo microarray were applied to the quantitative real-time PCR (qPCR), which was performed in duplicate to amplify the target and reference genes, using one step SYBR Prime-Script™ RT-PCR kit II (Catalog no. DRR086A, Takara, Japan) by Real-Time PCR (ABI 7900HT, Applied Biosystems, CA, USA). The sequences of primers and length of products were shown at Table 1. The reaction mixture (10.0 μL) contained 5.6 μL of freshly pre-mixed one step SYBR Green Real-Time PCR Master mix and Prime Script™ Enzyme Mix, 0.8 μmol/L of the primers, and 100 ng of RNA template. The qPCR program was designed with one cycle of 42 °C for 5 min, one cycle of 95 °C for 10 s, 40 cycles of 95 °C for 5 s and 60 °C for 34 s, followed by the dissociation step at 95 °C for 15 s, 60 °C for 60 s and 95 °C for 15 s. At the end of amplification, melting curve analysis was performed to identify amplification specificity. Amplification of ß-actin was used to normalize gene expression through the double standard curves method [11].
Table 1.
Primer sequences of genes selected for analysis by real-time RT-PCR
| Genes | GenBank accession | Primer sequence (5′ ~ 3′) | Product length (bp) | Tm (°C) |
|---|---|---|---|---|
| HSPA1L | NM_001123128.1 | F:CGCTTTGACCTGACTGGAAT | 120 | 60 |
| R:CTTGCCTGTGCTCTTGTCC | ||||
| CD8A | NM_001001907.1 | F:GCTGGACACCCGTTACATCT | 100 | 60 |
| R:CGAGCAGAAATAGTAGCCTTGG | ||||
| CD40 | NM_214194.1 | F:GGTTCGTCTGCCTCTGAAGT | 104 | 60 |
| R:GGCTGTTTGTTGGGTATTGG | ||||
| PSTPIP1 | NM_001244186.1 | F:CTCCTTTGACTCCCTGAAGC | 114 | 60 |
| R:TTCTGCCTCTCTCGGAACTC | ||||
| SLA-DQA1 | NM_001114062.2 | F:TGGACCTGGAGAAGAAGGAG | 132 | 60 |
| R:TGGAGCGTTTAGTCACGATG | ||||
| STAT2 | NM_213889.1 | F:TCCCAAATCACAAGGTTTCC | 109 | 60 |
| R:CAGATAGCCGAAGTCCCAAA | ||||
| GK | NM_001143708.1 | F:GCAGGTAGATGGAGGGATGA | 107 | 60 |
| R:CCAGGGCAGTTGTTTCAGG | ||||
| BMP7 | NM_001105290.1 | F:TCCAGGGCAAGCACAACT | 172 | 60 |
| R:TCGGTGAGGAAGTGGCTATC | ||||
| PIK3R5 | NM_213851.1 | F:CTGTCATTCCCTCCTTCCAA | 117 | 60 |
| R:GCCACCCTCCTCTTACTCTG | ||||
| SLA-DRB1 | NM_001113695.1 | F:TCTGCTCTTTGTTGCTGTGG | 120 | 60 |
| R:GGATGCTTGCTTGGAGTGTC | ||||
| THY1 | XM_005667396.1 | F:GGCATCGCTCTCTTGCTAAC | 125 | 60 |
| R:GGCAGGTTGGTGGTATTCTC | ||||
| TGFB1 | NM_214015.1 | F:AAGCGGCAACCAAATCTATG | 113 | 60 |
| R:CCCGAGAGAGCAATACAGGT | ||||
| SLA-1 | NM_001097431.1 | F:GTCAAGGAAACCGCACAGAT | 113 | 60 |
| R:CCCAAGTAGCAGCCAAACAT | ||||
| CD74 | NM_213774.1 | F:ATGGACGGTGTGAACTGGA | 100 | 60 |
| R:GAACCTCAAAGGGTGTCTCCT | ||||
| SOD2 | XM_005659113.1 | F:CCTTCACTTTGCCTCTTGGT | 127 | 60 |
| R:CACCGTTAGGGCTCAGATTT | ||||
| ACTA2 | XM_005671254.1 | F:GTCCACCTTCCAGCAAATGT | 105 | 60 |
| R:AGACAGCGAGCAGGGTAAGT | ||||
| SULT1E1 | NM_213992.1 | F:TGAAGTCTCATCTGCCACCT | 101 | 60 |
| R:AGAAACGACCACATCCTTGG | ||||
| β-actin | DQ845171.1 | F:GGCGCCCAGCACGAT | 66 | 60 |
| R:CCGATCCACACGGAGTACTTG |
HSPA1L heat shock 70 kDa protein 1-like, CD8A CD8a molecule (CD8A), CD40 CD40 molecule, TNF receptor superfamily member 5; SLA-DQA1 MHC class II histocompatibility antigen SLA-DQA, PSTPIP1 proline-serine-threonine phosphatase interacting protein 1, STAT2 signal transducer and activator of transcription 2, GK glycerol kinase, BMP7 bone morphogenetic protein 7, PIK3R5 phosphoinositide-3-kinase, regulatory subunit 5, SLA-DRB1 MHC class II histocompatibility antigen SLA-DRB1, THY1 Thy-1 cell surface antigen, TGFB1 transforming growth factor, beta 1, SLA-1 MHC class I antigen 1, CD74 CD74 molecule, major histocompatibility complex, class II invariant chain, SOD2 superoxide dismutase 2, mitochondrial, ACTA2 actin, alpha 2, smooth muscle, aorta, SULT1E1 sulfotransferase family 1E, estrogen-preferring, member 1
Statistical analysis
The detected data by samples from two fetuses each gilt were averaged and taken as one independent data involving into statistical analysis model. In addition to Oligo Microarray and qPCR data, all other data on growth performance, intestinal morphology and enzyme activities were analyzed via the t Student’s t test for a completely randomized design using SAS (SAS, Cary, NC). Results were expressed as the mean ± SD. Differences were considered to be significant when P <0.05, while a tendency was considered when 0.05 < P < 0.10.
Results
Growth performance
Feeding HF diet markedly increased the fetal weight (in average 585 g vs.508 g, P < 0.05) at d 90 of gestation.
Morphology and enzyme activities
Feeding HF diet tended to increase intestinal villous height (P = 0.055), but decrease crypt depth (P = 0.098) of fetus (Fig. 1). Meanwhile, the lactase activity was markedly increased (+55 %, P < 0.05) by feeding HF diet relative to CON diet, whereas the maltase activity did not markedly differ between groups (Fig. 2), and sucrase activity could not be detected in fetal intestine. Gene expression of digestive enzymes were not markedly differ between two groups (Additional file 1).
Fig. 1.
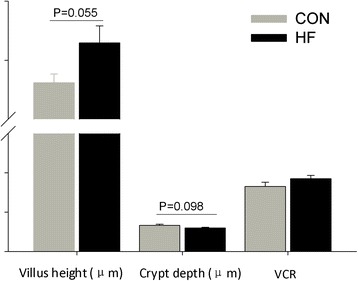
Effect of maternal high fat intake on the intestinal morphology of fetus (n = 4)
Fig. 2.
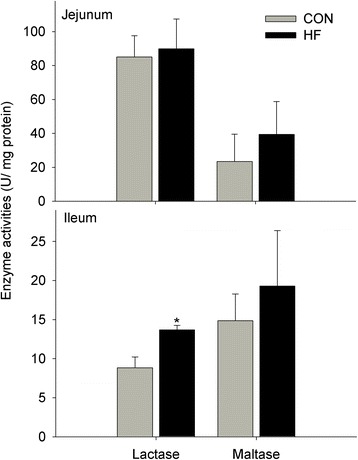
Effects of maternal high fat intake on digestive enzyme activities of fetal intestine (n = 4). The symbol “*” in figure represents there was significant difference at 5 % level (P < 0.05)
Differentially expressed genes in fetal intestine
A total of 61 genes were differentially expressed (at least 1.5 fold change, P < 0.05), and 39 genes were down-regulated while 22 genes were up-regulated (Table 2, Fig. 3). The changes in mRNA expression detected by porcine oligo microarrays were further validated by qRT-PCR (Table 3). Given their participation in crucial biological process and modulating signal pathways on immune response, cancer and metabolism, these genes were chosen for Real-Time PCR analysis.
Table 2.
Maternal high fat intake markedly regulated intestinal gene expressions related to immune response, signal transduction, cancer and metabolism
| Gene Symbola | Gene name | Fold changeb | P value |
|---|---|---|---|
| CCR7 | chemokine (C-C motif) receptor 7 | −2.94 | 0.023 |
| HSPA1L | heat shock 70 kDa protein 1-like | −2.50 | 0.016 |
| CD8A | CD8a molecule (CD8A) | −2.44 | 0.035 |
| CD3E | CD3e molecule, epsilon (CD3-TCR complex) (CD3E) | −2.27 | 0.033 |
| STK17B | serine/threonine kinase 17b | −2.00 | 0.026 |
| CD40 | CD40 molecule, TNF receptor superfamily member 5 | −2.00 | 0.011 |
| CD2 | CD2 molecule | −1.89 | 0.026 |
| SLA-DQA1 | MHC class II histocompatibility antigen SLA-DQA | −1.85 | 0.002 |
| PSTPIP1 | proline-serine-threonine phosphatase interacting protein 1 | −1.85 | 0.007 |
| SLAMF6 | SLAM family member 6 | −1.82 | 0.046 |
| TP53INP1 | tumor protein p53 inducible nuclear protein 1 | −1.82 | 0.000 |
| FAM78A | family with sequence similarity 78, member A | −1.79 | 0.015 |
| BCL2A1 | BCL2-related protein A1 | −1.79 | 0.023 |
| ARHGAP25 | Rho GTPase activating protein 25 | −1.75 | 0.011 |
| CD1.1 | CD1 antigen | −1.72 | 0.007 |
| STAT2 | signal transducer and activator of transcription 2 | −1.69 | 0.042 |
| ARHGAP30 | Rho GTPase activating protein 30 | −1.69 | 0.036 |
| BCL2A1 | BCL2-related protein A1 | −1.69 | 0.040 |
| IL10RB | interleukin 10 receptor, beta | −1.67 | 0.013 |
| GK | glycerol kinase | −1.64 | 0.041 |
| LTB | mRNA, clone:MLN010057G03, expressed in mesenteric lymph nodes | −1.64 | 0.031 |
| LCP1 | lymphocyte cytosolic protein 1 (L-plastin) | −1.61 | 0.014 |
| PGM1 | phosphoglucomutase 1 | −1.61 | 0.045 |
| NRROS | negative regulator of reactive oxygen species | −1.59 | 0.049 |
| CYTH4 | cytohesin 4 | −1.59 | 0.039 |
| BMP7 | bone morphogenetic protein 7 | −1.59 | 0.024 |
| PIK3R5 | phosphoinositide-3-kinase, regulatory subunit 5 | −1.56 | 0.009 |
| RGS14 | regulator of G-protein signaling 14 | −1.54 | 0.049 |
| GLRX | glutaredoxin (thioltransferase) | −1.54 | 0.025 |
| SLA-DRB1 | MHC class II histocompatibility antigen SLA-DRB1 | −1.52 | 0.028 |
| LPAR2 | lysophosphatidic acid receptor 2 | −1.52 | 0.016 |
| THY1 | Thy-1 cell surface antigen | −1.52 | 0.028 |
| TGFB1 | transforming growth factor, beta 1 | −1.52 | 0.022 |
| BAZ1A | bromodomain adjacent to zinc finger domain, 1A | −1.52 | 0.024 |
| CCDC69 | coiled-coil domain containing 69 | −1.49 | 0.048 |
| LRRK2 | leucine-rich repeat kinase 2 | −1.49 | 0.022 |
| SLA-1 | MHC class I antigen 1 | −1.49 | 0.018 |
| CD74 | CD74 molecule, major histocompatibility complex, class II invariant chain | −1.49 | 0.038 |
| SOD2 | superoxide dismutase 2, mitochondrial | 1.51 | 0.004 |
| ILF2 | interleukin enhancer binding factor 2 | 1.51 | 0.021 |
| CYP39A1 | cytochrome P450, family 39, subfamily A, polypeptide 1 | 1.52 | 0.043 |
| JPH4 | junctophilin 4 | 1.52 | 0.026 |
| ATCAY | ataxia, cerebellar, Cayman type | 1.53 | 0.008 |
| MATN2 | mRNA, clone:OVR010041A03, expressed in ovary | 1.53 | 0.016 |
| CRMP1 | Uncharacterized protein | 1.54 | 0.039 |
| RTDR1 | mRNA, clone:UTR010010H08, expressed in uterus. | 1.55 | 0.001 |
| SPARCL1 | SPARC-like 1 (hevin) | 1.56 | 0.035 |
| MATN2 | mRNA, clone:OVR010041A03, expressed in ovary | 1.56 | 0.019 |
| CCN2 | connective tissue growth factor | 1.57 | 0.042 |
| TUSC3 | mRNA, clone: HTMT10103A12, expressed in hypothalamus | 1.58 | 0.009 |
| ID4 | inhibitor of DNA binding 4, dominant negative helix-loop-helix protein | 1.58 | 0.024 |
| SPARC | secreted protein, acidic, cysteine-rich (osteonectin) | 1.60 | 0.035 |
| MEP1A | meprin A, alpha (PABA peptide hydrolase) | 1.63 | 0.018 |
| ARL10 | ADP-ribosylation factor-like 10 | 1.64 | 0.036 |
| STMN2 | stathmin-like 2 | 1.64 | 0.039 |
| ACTA2 | actin, alpha 2, smooth muscle, aorta | 1.66 | 0.016 |
| SHISA2 | shisa family member 2 | 1.76 | 0.029 |
| UCHL1 | ubiquitin carboxyl-terminal esterase L1 (ubiquitin thiolesterase) | 1.79 | 0.014 |
| OCRL | oculocerebrorenal syndrome of Lowe | 2.01 | 0.008 |
| SULT1E1 | sulfotransferase family 1E, estrogen-preferring, member 1 | 2.59 | 0.013 |
aGenes were selected from the Kyoto Encyclopedia of Genes and Genomes pathways related to intestinal immune response, signal transduction, cancer and metabolism (http://www.genome.jp/kegg/pathway.html)
bThe fold change was based on the ratio of HF group to CON group, n = 4 subpools/group
Fig. 3.
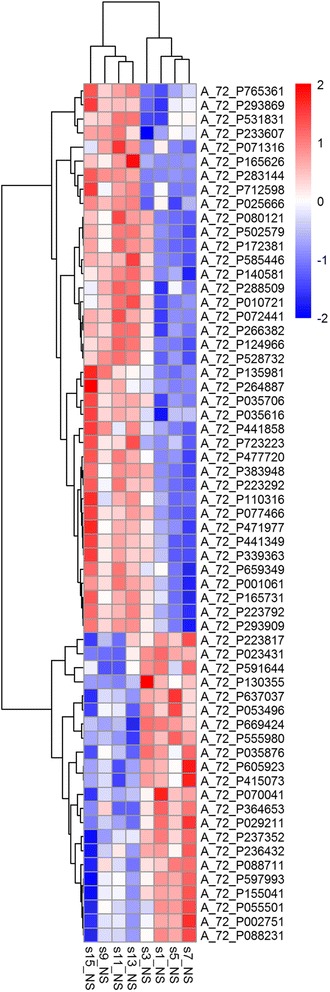
Heatmap of the 61 differentially expressed genes. The HF diet: s1_NS, s3-NS, s5-NS, s7-NS; The CON diet: s9_NS, s11-NS, s13-NS, s15-NS
Table 3.
Differentially expressed genes in fetal intestine by maternal high fat intake and validated by qPCR
| Fold changeb | |||
|---|---|---|---|
| Gene symbola | cDNA Microarray | qPCR | P value |
| ACTA2 | 1.66 | 1.10 | 0.246 |
| SULT1E1 | 2.59 | 1.88 | 0.002 |
| SOD2 | 1.51 | 1.75 | 0.036 |
| BMP7 | −1.59 | −1.09 | 0.722 |
| CD40 | −2.00 | −1.74 | 0.116 |
| CD74 | −1.49 | −1.36 | 0.029 |
| CD8A | −2.44 | −1.85 | 0.049 |
| GK | −1.64 | −1.33 | 0.041 |
| HSPAIL | −2.50 | −1.60 | 0.027 |
| PIK3R5 | −1.56 | −1.07 | 0.644 |
| PSTPIP1 | −1.85 | −1.48 | 0.083 |
| SLA-1 | −1.49 | −1.45 | 0.097 |
| SLA-DQA1 | −1.85 | −1.79 | 0.003 |
| SLA-DRB1 | −1.52 | −1.33 | 0.136 |
| STAT2 | −1.69 | −1.18 | 0.125 |
| TGF-β | −1.52 | −1.19 | 0.296 |
| THY1 | −1.52 | −1.29 | 0.118 |
aGenes were selected on the basis of their crucial role on regulating intestinal immune response (i.e.SLA-DRB1,SLA-DQA,HSPA1L,CD74,CD40), colorectal cancer (i.e.TGF-β,PIK3R5), signal transduction (i.e. PSTPIP1,BMP7,STAT2) and metabolism (i.e.GK, SULT1E1). These genes by DNA microarray were all significantly regulated (P < 0.05, at least 1.5 fold change)
bThe fold change was based on the ratio of HF group to CON group, n = 4 subpools/group
Analysis of gene ontology and signal pathway
The differentially expressed genes were clustered according to their biological process ontology by Gene Ontology (GO) analysis from the SBS analysis system (http://www.shanghaibiotech.com/). A large number of these genes were associated with antigen processing and presentation [i.e. D74, CD8A, SLA-DOB, SLA-DRB1, SLA-DQA, HSPA1L], intestinal immune network for IgA production [i.e. CD40, IL6, TGFβ1], Jak-STAT signaling pathway [i.e. IL6, STAT2 and PIK3R5], TGF-ß signaling pathway [i.e. TGF-β and PIK3R5], pathways in cancer [i.e. LEF1, PIK3R5, NOS2] and glycerolipid metabolism [i.e. GK, PNLIPRP1] et al. (Table 2, Fig. 4).
Fig. 4.
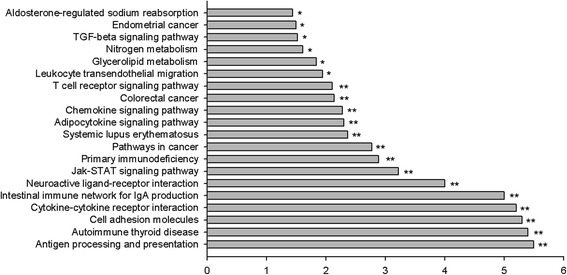
Signal pathway enrichment analysis of fetal intestine by HF diet relative to CON diet (n = 4 subpools/group). The pathway terms were according to the down-regulated genes for certain biological processes, enriched categories are those identified as significantly enriched after multiple testing. * P < 0.05, ** P < 0.01. The value by horizontal axis resulted from negative value of Log (enrichment test P value, base 10)
Consequently, maternal HF intake markedly modified 33 signal pathways (P < 0.01) (Table 4), which were mainly involved in immune response (i.e. antigen processing and presentation, intestinal immune network for IgA production, primary immunodeficiency), signaling transduction (i.e. TGF-ß signaling pathway, chemokine signaling pathway), cancer (i.e. colorectal cancer, pathways in cancer), metabolism (i.e. glycerolipid metabolism, nitrogen metabolism), signaling molecules and interaction (i.e. cytokine-cytokine receptor interaction, cell adhesion molecules, neuroactive ligand-receptor interaction).
Table 4.
The markedly modified signal pathways in fetal intestine of gilts fed HF diet
| Name | Hits a | Total b | Percent | Enrichment test |
|---|---|---|---|---|
| p value | ||||
| Allograft rejection | 6 | 34 | 17.65 % | 0.000 |
| Antigen processing and presentation | 8 | 64 | 12.50 % | 0.000 |
| Autoimmune thyroid disease | 6 | 45 | 13.33 % | 0.000 |
| Cell adhesion molecules | 8 | 71 | 11.27 % | 0.000 |
| Cytokine-cytokine receptor interaction | 10 | 142 | 7.04 % | 0.000 |
| Hematopoietic cell lineage | 7 | 63 | 11.11 % | 0.000 |
| Intestinal immune network for IgA production | 7 | 48 | 14.58 % | 0.000 |
| Leishmania infection | 8 | 63 | 12.70 % | 0.000 |
| Viral myocarditis | 9 | 46 | 19.57 % | 0.000 |
| Graft-versus-host disease | 6 | 57 | 10.53 % | 1E-04 |
| Neuroactive ligand-receptor interaction | 10 | 174 | 5.75 % | 1E-04 |
| Type I diabetes mellitus | 5 | 40 | 12.50 % | 2E-04 |
| Asthma | 5 | 50 | 10.00 % | 5E-04 |
| Jak-STAT signaling pathway | 6 | 82 | 7.32 % | 6E-04 |
| Primary immunodeficiency | 4 | 37 | 10.81 % | 0.0013 |
| Pathways in cancer | 7 | 140 | 5.00 % | 0.0017 |
| Hypertrophic cardiomyopathy | 4 | 43 | 9.30 % | 0.0022 |
| Systemic lupus erythematosus | 5 | 86 | 5.81 % | 0.0043 |
| Adipocytokine signaling pathway | 4 | 55 | 7.27 % | 0.005 |
| Chemokine signaling pathway | 5 | 90 | 5.56 % | 0.0052 |
| Colorectal cancer | 3 | 31 | 9.68 % | 0.0072 |
| Fc gamma R-mediated phagocytosis | 3 | 32 | 9.38 % | 0.0078 |
| T cell receptor signaling pathway | 4 | 63 | 6.35 % | 0.0078 |
| Leukocyte transendothelial migration | 4 | 71 | 5.63 % | 0.0115 |
| Acute myeloid leukemia | 3 | 39 | 7.69 % | 0.0129 |
| Dilated cardiomyopathy | 3 | 40 | 7.50 % | 0.0137 |
| Glycerolipid metabolism | 3 | 41 | 7.32 % | 0.0146 |
| Arrhythmogenic right ventricular cardiomyopathy | 3 | 47 | 6.38 % | 0.0205 |
| Nitrogen metabolism | 2 | 19 | 10.53 % | 0.0246 |
| TGF-beta signaling pathway | 3 | 55 | 5.45 % | 0.0302 |
| Endometrial cancer | 2 | 22 | 9.09 % | 0.0316 |
| Aldosterone-regulated sodium reabsorption | 2 | 24 | 8.33 % | 0.0366 |
| Type II diabetes mellitus | 2 | 24 | 8.33 % | 0.0366 |
aHits mean the number of differential expressed genes within the particular GO term
bTotal: the total number of genes within the particular GO term
Discussion
Some studies have indicated that maternal nutrition would affect the intestinal development and function of offspring [4, 13–15].
In this study, maternal high fat intake increased intestinal villous height and lactase activity, which is similar as our recent study that maternal over-nutrition markedly increased birth weight, accordingly intestinal morphology as well as lactase activity [4]. It may be rational that the heavier birth weight needs higher lactase activity in preparation for better degradation of lactose, which is a crucial energy source in neonatal period [16]. However, a recent study indicated that maternal high fat intake would induce intestinal inflammation and poor gut barrier function in the offspring of mice [5]. In this study, porcine oligo miacro array analysis was used to determine the genomic response of intestine to maternal high fat intake, in an attempt to reveal the potential mechanism. According to the strict selection criteria, we found a total of 61 genes were differentially regulated and 64 % of them (39 genes) was down-regulated by HF diet. With the bioinformatics analysis, these down-regulated genes were mainly involved in process of immune response, signaling transduction, pathways in cancer and metabolism, suggesting the inhibitory effects of maternal high fat intake on certain biological events. The maternal diet fat composition could change the maternal-to-fetal fatty acid transfer and intestinal membrane n-6 and n-3 fatty acids composition of newborns, thus altering intestinal function [13]. In this study, therefore, it is rational that the addition of soy oil in maternal diet would induce alterations in intestinal physiology of fetus. Obviously, antigen processing and presentation in intestine could be inhibited by feeding HF diet, as indicated by the markedly decreasing gene expressions (i.e. SLA-1, SLA -DRB1, SLA-DQA1, CD74 and CD8, 1.5 ~ 2.5 fold reduction). Particularly, SLA-1, SLA -DRB1 and SLA-DQA1 are belonged to the highly polymorphic swine leucocyte antigen genes, which determine the immune response to disease and vaccine [17]. Among them, SLA-1 could interact with natural killer cells to prevent cytotoxicity [18], while SLA-DRB1 and -DQA1 mainly present exogenous peptides for T cells [18, 19]. Previous studies have shown that maternal high fat intake impaired intestinal barrier and immune system through altering immune cell homeostasis, such as the number of T cells and macrophages [13]. Furthermore, intestinal immune network for IgA production may be impaired by HF diet, as shown by the decreasing gene expression of CD40, IL-6 and TGF-ß. These genes are required for B cells proliferation and differentiation in Peyer’s patches, their down-regulation would reduce the homing of T cells and IgA+ plasma cells to the intestine, thus impair the immune homeostasis of intestine [20, 21].
Several signal transduction pathways related to inflammatory and immune response were affected by maternal high fat intake. For example, the TGF-β signaling pathway was affected by HF diet, as indicated by the decreasing gene expression of TGF-β and Bmp7 (approximately 1.6 fold reduction). TGF-β is a multifunctional factor regulating cell growth, adhesion and differentiation [22, 23], also exerting anti-inflammatory effects by inhibiting NF-κB expression in the intestinal epithelium [24]. The oral administration of TGF-β has been shown to decrease severity and incidence of necrotizing enterocolitis in neonatal rat necrotizing enterocolitis model [24]. In addition, feeding HF diet affected intestinal Jak-STAT signaling pathway, as shown by the decreasing gene expression of IL6, STAT2 and PIK3R5. The Jak-STAT signaling pathway is required for T cell differentiation, B cell maturation and secretion of sIgA [25], these down-regulated genes by HF diet may induce the abnormal intestinal innate immune response. Similarly, previous study demonstrated that maternal high protein diet would decrease liver mass, associated with altering gene expressions mapping to Jak-STAT signaling pathway in mouse offspring [26].
Furthermore, the lower expressions of TGF-β and PIK3R5 genes by HF diet may affect the progression of colorectal cancer. TGF-β1/Smads signaling pathway was demonstrated to mediate epithelial-to-mesenchymal transition, associated with the progression of colorectal cancer [27]. Mutation of PIK3R5 and other genes (i.e. PRKCZ, PTEN, RHEB and RPS6KB1) have altered PI3K signaling pathway, which is the central pathway for both colorectal and breast cancers [28]. Recent studies also indicated that maternal high fat diet would modify the susceptibility to breast cancer [29, 30], meanwhile it is dependent on fat or oil sources [31–33].
In this study, moreover, the markedly reduced glycerol kinase by feeding HF diet suggests the intestinal metabolism was altered. Glycerol kinase is required to release glycerol from glycerol-3-phosphate and dihydroxyacetone, and intestinal glycerol could produce 20 ~ 25 % of total endogenous glucose under insulinopenia, suggesting the important role of glycerol in intestinal metabolism [34]. Although most of genes were markedly down-regulated by HF diet, some of genes (SOD2, CYP39A1, CCN2, SPARC et al.) were up-regulated. Particularly, SOD2, as an anti-oxidative enzyme in living cells, was highly expressed (1.75 fold change, P = 0.04). Likewise, a recent study demonstrated that maternal high energy intake increased the expression of SOD in offspring ileum [5]. Previous study indicated that the increasing SOD gene is not necessarily associated with a better antioxidant capability, for example, the inflamed intestinal mucosa has been shown to contain higher SOD protein compared with normal tissues [35]. In addition, we found that feeding HF diet markedly increased gene expression of SULT1E by both DNA Microarray and RT-PCR analysis. SULT1E, as an estrogen-preferring drug metabolizing enzyme, its highly expression may be an compensatory response to high circulating estrogen, which occurs in dams fed high fat diet [36]. It has been shown that the estrogen deletion by SULT1E over-expression is associated with the risk of developing different types of cancers [28, 37].
Conclusion
In summary, maternal high fat intake was able to increase fetal and intestinal weights as well as lactase activity, however, it altered the intestinal immune response, signal transduction and metabolism.
Acknowledgements
We would like to thank the staff at our laboratory for their ongoing assistance.
Funding
The present study was supported by the National Natural Science Foundation of China (31101727) and the National Basic Research Program (973 Program) of China (No. 2013CB127306).
Additional file
Effect of maternal high fat intake on gene expression of digestive enzymes in fetal intestine. (DOCX 34 kb)
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
The authors’ contributions are as follows: L-QC contributed to the study design and manuscript preparation; PLL, ZGY and LC carried out the study; LH, LLQ and RW contributed to the sample analysis; ZFF, YL, JL and SYX contributed to the data analysis; BF and DW contributed to the data interpretation. All authors read and approved the final manuscript.
References
- 1.Wu G, Bazer FW, Wallace JM, Spencer TE. Board-invited review: intrauterine growth retardation: implications for the animal sciences. J Anim Sci. 2006;84:2316–2337. doi: 10.2527/jas.2006-156. [DOI] [PubMed] [Google Scholar]
- 2.Sangild PT. Gut responses to enteral nutrition in preterm infants and animals. Exp Biol Med (Maywood) 2006;231:1695–1711. doi: 10.1177/153537020623101106. [DOI] [PubMed] [Google Scholar]
- 3.Pinheiro DF, Pinheiro PF, Buratini J, Jr, Castilho AC, Lima PF, Trinca LA, Vicentini-Paulino Mde L. Maternal protein restriction during pregnancy affects gene expression and immunolocalization of intestinal nutrient transporters in rats. Clin Sci (Lond) 2013;125:281–289. doi: 10.1042/CS20120400. [DOI] [PubMed] [Google Scholar]
- 4.Cao M, Che L, Wang J, Yang M, Su G, Fang Z, Lin Y, Xu S, Wu D. Effects of maternal over- and undernutrition on intestinal morphology, enzyme activity, and gene expression of nutrient transporters in newborn and weaned pigs. Nutrition. 2014;30:1442–1447. doi: 10.1016/j.nut.2014.04.016. [DOI] [PubMed] [Google Scholar]
- 5.Xue Y, Wang H, Du M, Zhu MJ. Maternal obesity induces gut inflammation and impairs gut epithelial barrier function in nonobese diabetic mice. J Nutr Biochem. 2014;25:758–764. doi: 10.1016/j.jnutbio.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yan X, Huang Y, Wang H, Du M, Hess BW, Ford SP, Nathanielsz PW, Zhu MJ. Maternal obesity induces sustained inflammation in both fetal and offspring large intestine of sheep. Inflamm Bowel Dis. 2011;17:1513–1522. doi: 10.1002/ibd.21539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gonzalez-Bulnes A, Astiz S, Ovilo C, Lopez-Bote CJ, Sanchez-Sanchez R, Perez-Solana ML, Torres-Rovira L, Ayuso M, Gonzalez J. Early-postnatal changes in adiposity and lipids profile by transgenerational developmental programming in swine with obesity/leptin resistance. J Endocrinol. 2014;223:M17–29. doi: 10.1530/JOE-14-0217. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Q, Widmer G, Tzipori S. A pig model of the human gastrointestinal tract. Gut Microbes. 2013;4:193–200. doi: 10.4161/gmic.23867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heinritz SN, Mosenthin R, Weiss E. Use of pigs as a potential model for research into dietary modulation of the human gut microbiota. Nutr Res Rev. 2013;26:191–209. doi: 10.1017/S0954422413000152. [DOI] [PubMed] [Google Scholar]
- 10.Han F, Hu L, Xuan Y, Ding X, Luo Y, Bai S, He S, Zhang K, Che L. Effects of high nutrient intake on the growth performance, intestinal morphology and immune function of neonatal intra-uterine growth-retarded pigs. Br J Nutr. 2013;110:1819–1827. doi: 10.1017/S0007114513001232. [DOI] [PubMed] [Google Scholar]
- 11.Hu L, Liu Y, Yan C, Peng X, Xu Q, Xuan Y, Han F, Tian G, Fang Z, Lin Y, et al. Postnatal nutritional restriction affects growth and immune function of piglets with intra-uterine growth restriction. Br J Nutr. 2015;114:53–62. doi: 10.1017/S0007114515001579. [DOI] [PubMed] [Google Scholar]
- 12.Che L, Chen H, Yu B, He J, Zheng P, Mao X, Yu J, Huang Z, Chen D. Long-term intake of pea fiber affects colonic barrier function, bacterial and transcriptional profile in pig model. Nutr Cancer. 2014;66:388–399. doi: 10.1080/01635581.2014.884229. [DOI] [PubMed] [Google Scholar]
- 13.Innis SM, Dai C, Wu X, Buchan AM, Jacobson K. Perinatal lipid nutrition alters early intestinal development and programs the response to experimental colitis in young adult rats. Am J Physiol Gastrointest Liver Physiol. 2010;299:G1376–1385. doi: 10.1152/ajpgi.00258.2010. [DOI] [PubMed] [Google Scholar]
- 14.Boudry G, Douard V, Mourot J, Lalles JP, Le Huerou-Luron I. Linseed oil in the maternal diet during gestation and lactation modifies fatty acid composition, mucosal architecture, and mast cell regulation of the ileal barrier in piglets. J Nutr. 2009;139:1110–1117. doi: 10.3945/jn.108.102640. [DOI] [PubMed] [Google Scholar]
- 15.Desaldeleer C, Ferret-Bernard S, de Quelen F, Le Normand L, Perrier C, Savary G, Rome V, Michel C, Mourot J, Le Huerou-Luron I, Boudry G. Maternal 18:3n-3 favors piglet intestinal passage of LPS and promotes intestinal anti-inflammatory response to this bacterial ligand. J Nutr Biochem. 2014;25:1090–1098. doi: 10.1016/j.jnutbio.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 16.Heyman MB, Committee on N Lactose intolerance in infants, children, and adolescents. Pediatrics. 2006;118:1279–1286. doi: 10.1542/peds.2006-1721. [DOI] [PubMed] [Google Scholar]
- 17.Ho CS, Lunney JK, Lee JH, Franzo-Romain MH, Martens GW, Rowland RR, Smith DM. Molecular characterization of swine leucocyte antigen class II genes in outbred pig populations. Anim Genet. 2010;41:428–432. doi: 10.1111/j.1365-2052.2010.02019.x. [DOI] [PubMed] [Google Scholar]
- 18.Lunney JK, Ho CS, Wysocki M, Smith DM. Molecular genetics of the swine major histocompatibility complex, the SLA complex. Dev Comp Immunol. 2009;33:362–374. doi: 10.1016/j.dci.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 19.Summerfield A, Guzylack-Piriou L, Schaub A, Carrasco CP, Tache V, Charley B, McCullough KC. Porcine peripheral blood dendritic cells and natural interferon-producing cells. Immunology. 2003;110:440–449. doi: 10.1111/j.1365-2567.2003.01755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang D, Dubois RN, Richmond A. The role of chemokines in intestinal inflammation and cancer. Curr Opin Pharmacol. 2009;9:688–696. doi: 10.1016/j.coph.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chu VT, Beller A, Rausch S, Strandmark J, Zanker M, Arbach O, Kruglov A, Berek C. Eosinophils promote generation and maintenance of immunoglobulin-A-expressing plasma cells and contribute to gut immune homeostasis. Immunity. 2014;40:582–593. doi: 10.1016/j.immuni.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 22.Massagué J. TGFβ in cancer. Cell. 2008;134:215–230. doi: 10.1016/j.cell.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van’t Land B, Meijer H, Frerichs J, Koetsier M, Jager D, Smeets R, M'Rabet L, Hoijer M. Transforming Growth Factor-β2 protects the small intestine during methotrexate treatment in rats possibly by reducing stem cell cycling. Br J Cancer. 2002;87:113–8. [DOI] [PMC free article] [PubMed]
- 24.Shiou SR, Yu Y, Guo Y, Westerhoff M, Lu L, Petrof EO, Sun J, Claud EC. Oral administration of transforming growth factor-beta1 (TGF-beta1) protects the immature gut from injury via Smad protein-dependent suppression of epithelial nuclear factor kappaB (NF-kappaB) signaling and proinflammatory cytokine production. J Biol Chem. 2013;288:34757–34766. doi: 10.1074/jbc.M113.503946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heneghan AF, Pierre JF, Kudsk KA. JAK-STAT and intestinal mucosal immunology. JAKSTAT. 2013;2:e25530. doi: 10.4161/jkst.25530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vanselow J, Kucia M, Langhammer M, Koczan D, Rehfeldt C, Metges CC. Hepatic expression of the GH/JAK/STAT/IGF pathway, acute-phase response signalling and complement system are affected in mouse offspring by prenatal and early postnatal exposure to maternal high-protein diet. Eur J Nutr. 2011;50:611–623. doi: 10.1007/s00394-011-0168-5. [DOI] [PubMed] [Google Scholar]
- 27.Ji Q, Liu X, Han Z, Zhou L, Sui H, Yan L, Jiang H, Ren J, Cai J, Li Q. Resveratrol suppresses epithelial-to-mesenchymal transition in colorectal cancer through TGF-β1/Smads signaling pathway mediated Snail/E-cadherin expression. BMC Cancer. 2015;15:1. doi: 10.1186/1471-2407-15-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wood LD, Parsons DW, Jones S, Lin J, Sjöblom T, Leary RJ, Shen D, Boca SM, Barber T, Ptak J. The genomic landscapes of human breast and colorectal cancers. Science. 2007;318:1108–1113. doi: 10.1126/science.1145720. [DOI] [PubMed] [Google Scholar]
- 29.Montales MT, Melnyk SB, Simmen FA, Simmen RC. Maternal metabolic perturbations elicited by high-fat diet promote Wnt-1-induced mammary tumor risk in adult female offspring via long-term effects on mammary and systemic phenotypes. Carcinogenesis. 2014;35:2102–2112. doi: 10.1093/carcin/bgu106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Oliveira AF, Fontelles CC, Rosim MP, de Oliveira TF, de Melo Loureiro AP, Mancini-Filho J, Rogero MM, Moreno FS, de Assis S, Barbisan LF, et al. Exposure to lard-based high-fat diet during fetal and lactation periods modifies breast cancer susceptibility in adulthood in rats. J Nutr Biochem. 2014;25:613–622. doi: 10.1016/j.jnutbio.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mabasa L, Cho K, Walters MW, Bae S, Park CS. Maternal dietary canola oil suppresses growth of mammary carcinogenesis in female rat offspring. Nutr Cancer. 2013;65:695–701. doi: 10.1080/01635581.2013.789539. [DOI] [PubMed] [Google Scholar]
- 32.Czuba AK, Mazurkiewicz M, Stanimirova-Daszykowska I. Enrichment of maternal diet with conjugated linoleic acids influences desaturases activity and fatty acids profile in livers and hepatic microsomes of the offspring with 7, 12-dimethylbenz [a] anthracene-induced mammary tumors. Acta Pol Pharm. 2014;71:747. [PubMed] [Google Scholar]
- 33.Su H-M, Hsieh P-H, Chen H-F. A maternal high n-6 fat diet with fish oil supplementation during pregnancy and lactation in rats decreases breast cancer risk in the female offspring. J Nutr Biochem. 2010;21:1033–1037. doi: 10.1016/j.jnutbio.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 34.Croset M, Rajas F, Zitoun C, Hurot J-M, Montano S, Mithieux G. Rat small intestine is an insulin-sensitive gluconeogenic organ. Diabetes. 2001;50:740–746. doi: 10.2337/diabetes.50.4.740. [DOI] [PubMed] [Google Scholar]
- 35.Kruidenier L, Kuiper I, van Duijn W, Marklund SL, van Hogezand RA, Lamers CB, Verspaget HW. Differential mucosal expression of three superoxide dismutase isoforms in inflammatory bowel disease. J Pathol. 2003;201:7–16. doi: 10.1002/path.1407. [DOI] [PubMed] [Google Scholar]
- 36.Hilakivi-Clarke L, Clarke R, Onojafe I, Raygada M, Cho E, Lippman M. A maternal diet high in n − 6 polyunsaturated fats alters mammary gland development, puberty onset, and breast cancer risk among female rat offspring. Proc Natl Acad Sci. 1997;94:9372–9377. doi: 10.1073/pnas.94.17.9372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sharda DR, Miller-Lee JL, Kanski GM, Hunter JC, Lang CH, Kennett MJ, Korzick DH. Comparison of the agar block and Lieber–DeCarli diets to study chronic alcohol consumption in an aging model of Fischer 344 female rats. J Pharmacol Toxicol Methods. 2012;66:257–263. doi: 10.1016/j.vascn.2012.08.166. [DOI] [PMC free article] [PubMed] [Google Scholar]


