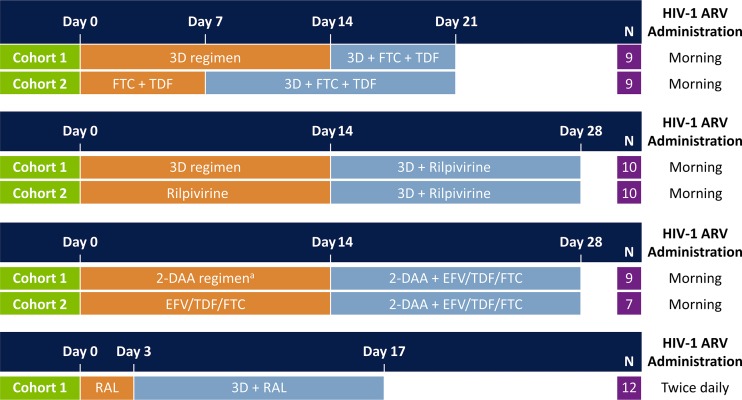
FIG 1.
Study design of drug-drug interaction studies. All study drugs were administered under nonfasting conditions. Paritaprevir/ritonavir and ombitasvir once-daily doses were administered in the morning, and dasabuvir was administered in the morning and evening. The drug-drug interaction study of efavirenz (EFV)/tenofovir disoproxil fumarate (TDF)/emtricitabine (FTC) (i.e., Atripla) with direct-acting antiviral (DAA) agents was conducted with the 2-DAA regimen of paritaprevir/ritonavir and dasabuvir. 3D, paritaprevir/ritonavir, ombitasvir, and dasabuvir; ARV, antiretroviral; RAL, raltegravir.