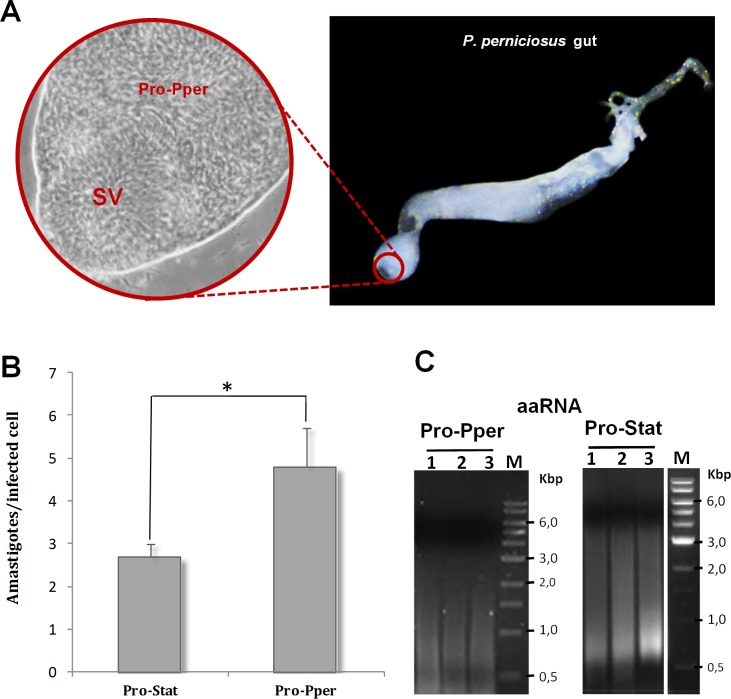
Fig 1. Sand fly gut dissection, in vitro infectivity and mRNA amplification.
(A) Detail of Pro-Pper (40X) within the anterior thoracic midgut (10X). SV: stomodeal valve. Sand flies of an established colony were dissected to extract the whole guts. After that, the anterior part of the thoracic midgut, behind the SV, was separated in a PBS drop and slightly squeezed with a coverslip. Then, the PBS drop containing Pro-Pper in suspension was recovered, thus avoiding carryover of gut tissue as much as possible. (B) The U937 cell line was differentiated with phorbol esters on 8-well chamber slides and in vitro infected with L. infantum Pro-Pper and Pro-Stat (approximately 5 x 104 promastigotes at a phagocyte:promastigote ratio 1:5 were added). The preparations were fixed and stained with modified Giemsa and 100 cells were randomly counted per replicate. The average number of amastigotes per infected cell was measured at 48 h post-infection. Mean ± SD: 2.7 ± 0.4 in the case of Pro-Stat and 4.8 ± 0.9 in the case of Pro-Pper. (C) Agarose gel electrophoresis of aaRNA samples used for the microarray analysis after synthesis of labeled cDNA. Total RNA was purified from isolated Pro-Pper immediately after dissection and doubly amplified (aaRNA) with MessageAmpII aRNA Amplification Kit (Life Technologies), due to sample amount requirements. Pro-Stat RNA was isolated and processed following the same procedure as for Pro-Pper. Five μl aliquots of the aaRNA samples were run at 5 V/cm in a 1.5% agarose gel prepared with RNase-free water after treatment of the electrophoresis cell, tray and comb with hydrogen peroxide. Three biological replicates of the microarray hybridization experiment were performed.