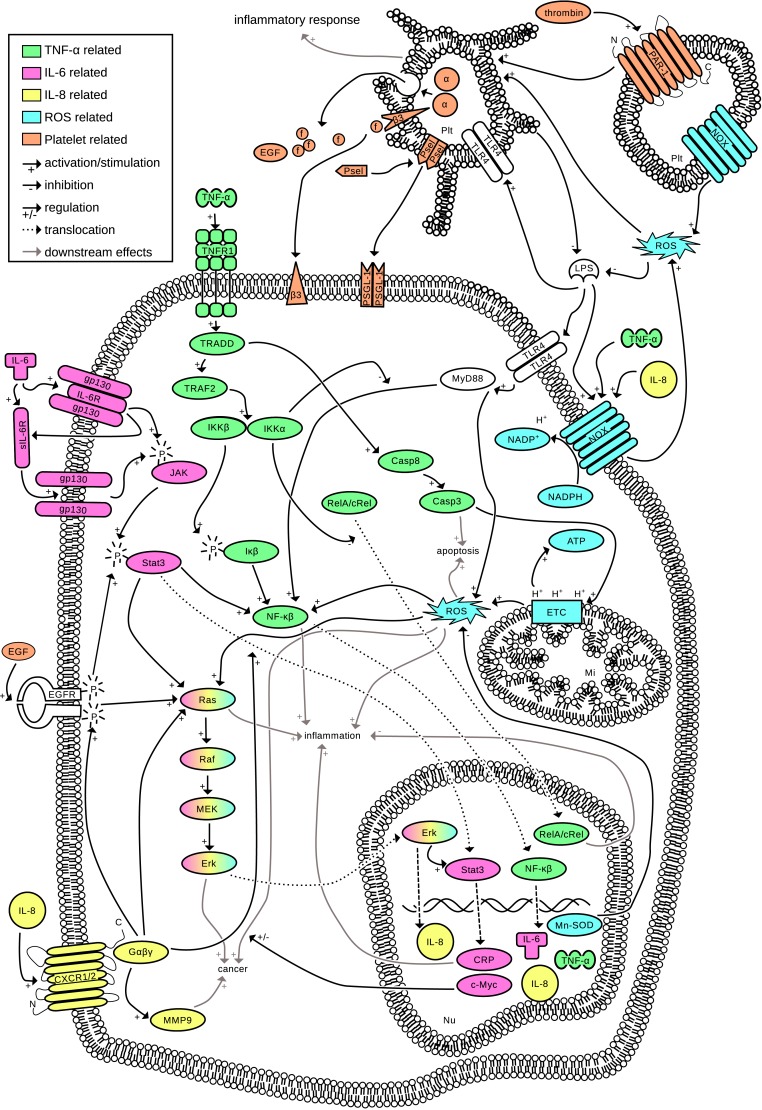
Fig 1. Interconnection of inflammatory pathways.
Five important processes in inflammation are combined into one interconnected pathway network. (TNF-α, green) TNF-α is part of the very extensive NF-κβ pathway [3]. TNF-α starts multiple signaling cascades by recruiting the tumor necrosis factor receptor 1 (TNFR1), which is subsequently recruiting the TNFR1 associated death domain (TRADD) [4]. TRADD on one side activates the caspase cascade which leads to apoptosis and ROS production [5]. On the other side, the core component complex IKKα/β (Iκα/β kinase) of the NF-κβ pathway is activated. The β part subsequently phosphorylates Iκβ which in turn activates NF-κβ [4]. This leads to translocation of NF-κβ dimers to the nucleus and upregulation of (among others) IL-6, IL-8, TNF-α, and manganese superoxide dismutase (Mn-SOD) [4–6]. (IL-6, pink) IL-6 is an important activator of the Janus kinase signal transducer and an activator of transcription [7]. The JAK/Stat pathway is involved in the upregulation of pro-inflammatory cytokines in inflammation, cell proliferation and tumorigenesis [6,8]. IL-6 binds to the IL-6 receptor (IL-6R), which in turn associates with the gp130 protein complex on the cell membrane and phosphorylates JAK. Only a few cell types express the IL-6R on the cell membrane, however, all cells have a soluble form of this receptor (sIL-6R) and the gp130 dimer, meaning that JAK/Stat signaling can be activated in essentially all cell types. The complexation of IL-6 with gp130 and consequently the phosphorylation of Stat3 is needed for a controlled inflammatory response [9,10]. Activated Stat3 dimerizes and translocates to the nucleus, where (among others) c-Myc and c-reactive protein (CRP) are upregulated [10]. Furthermore, activated Stat3 stimulates NF-κβ [2] and the Ras oncogene which is important in both the development of cancer [6,10] and stimulation of inflammation [6]. (IL-8, yellow) IL-8 is a pro-inflammatory chemokine whose expression is primarily regulated by NF-κβ. IL-8 binds to G-coupled protein receptor CXCR1/2, which in turn stimulates the Ras oncogene and promotes the nuclear translocation of Stat3 [11]. It is the most powerful human neutrophil chemoattractant and stimulates tumor growth. Furthermore, TNF-α and ROS are potent inducers of IL-8 production [12]. (ROS, blue) At an inflammatory site, ROS (which include superoxide radicals, nitric oxide and hydrogen peroxide [5]) are produced continuously (the oxidative burst) as one of the first lines of attack against pathogens [13]. ROS production is vital in acute inflammation, however, a too high production of ROS can cause DNA repair failure [6] and modifications in proteins [13], and are carcinogenic [5]. Intracellularly, most ROS are produced by the mitochondrial electron transport chain (ETC), which is also stimulated in response to TNF-α [5]. These ROS are important for apoptosis as well as cell maintenance, but also stimulate NF-κβ, inflammation and cancer [13]. ROS can also activate platelets [14]. (Platelets, orange) Platelets are derived from megakaryocytes, do not have a nucleus and are essential for hemostasis and thrombosis. However, platelets are also loaded with immune modulators, and can drive the inflammatory response. Platelets express NADPH oxidase (NOX) and are an important source of ROS [15]. Upon activation by thrombin or ROS, α-granules are secreted which contain (among others) fibrinogen, P-selectin and EGF (15). f = fibrinogen, Psel = P-selectin, EGF = endothelial growth factor, α = α-granules, β3 = β-integrin receptors, LPS = lipopolysaccharide, Plt = platelet, Nu = nucleus.