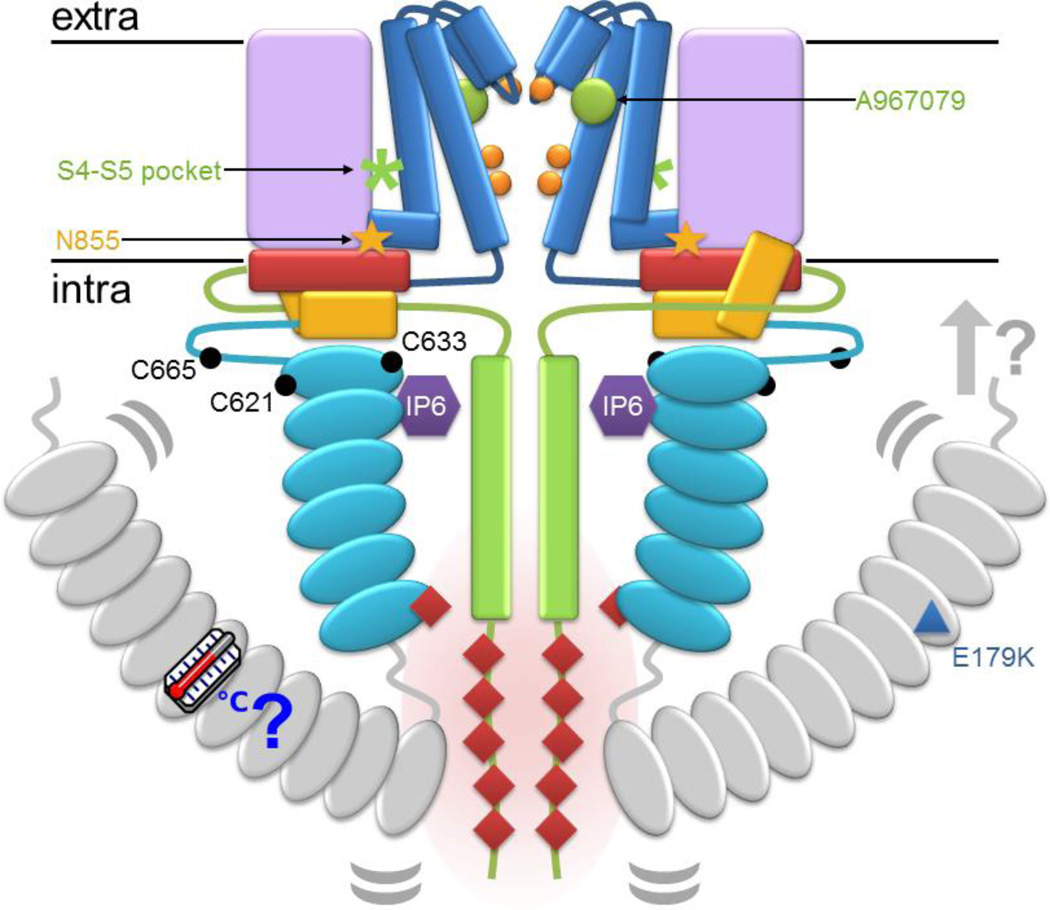
Figure 1.
A schematic of TRPA1 maps a number of demonstrated and proposed regulatory handles. The schematic follows the color scheme from N- to C-terminus: ankyrin repeats, turquoise; linker, yellow; S1–S4, lilac; S5–S6, blue; gate residues, orange; TRP-domain helix, red; and C-terminus, green. The regions absent in the structure are shown here in grey. The antagonist A967079 is represented as a green circle and the S4–S5 pocket is marked with a green asterisk. The positions of cysteines essential for response to reactive electrophiles are marked as black circles in the membrane-proximal N-terminal region that surrounds the TRP-domain helix (red). The position of the familial episodic pain variation N855S and the paradoxical heat syndrome variation E179K are marked with a yellow star and blue triangle, respectively. IP6 is represented as a purple hexagon between the membrane-proximal ankyrin repeats (turquoise) and coiled coil (green). The N-terminus and ankyrin repeats 1–11, which are missing from the TRPA1 structure, are in grey. Curved grey lines denote the suspected flexibility of the linkage between 1–11 and the remaining ankyrin repeats. Potential interactions between the N-terminus and the membrane or membrane-associated factors are indicated by a grey arrow. Intracellular mutations reported to disturb calcium-dependent potentiation or inactivation of TRPA1 are marked with red diamonds, which cluster at the intersection of the ARD and the C-terminus.