FIG 3.
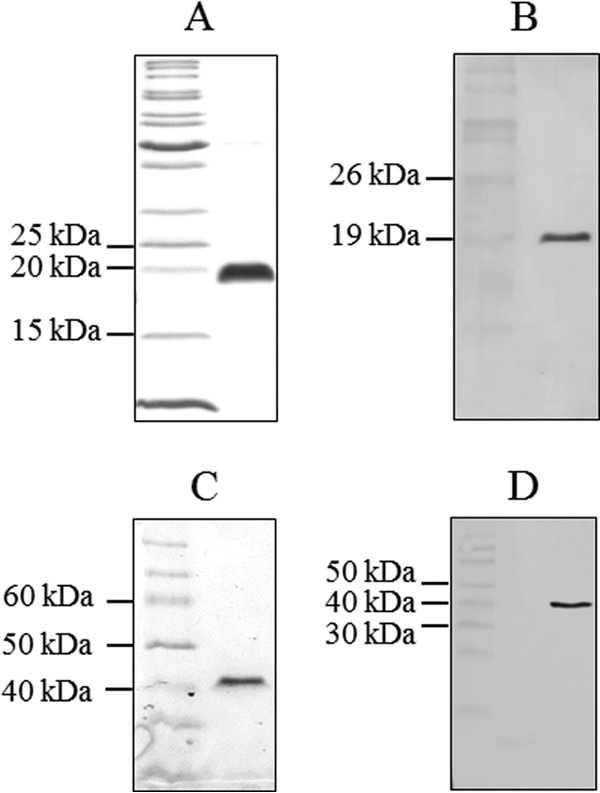
Purification of donor strand-complemented CfaB and CfaE subunits. The identity and purity of donor strand-complemented CfaB (A, B) and CfaE (C, D) were confirmed by SDS-PAGE (A, C) and immunoblotting with an antihexahistidine antibody (B, D). The electrophoretic migrations of CfaB and CfaE fusion proteins were consistent with their predicted molecular sizes of 18.6 and 41 kDa, respectively.
