Figure 2.
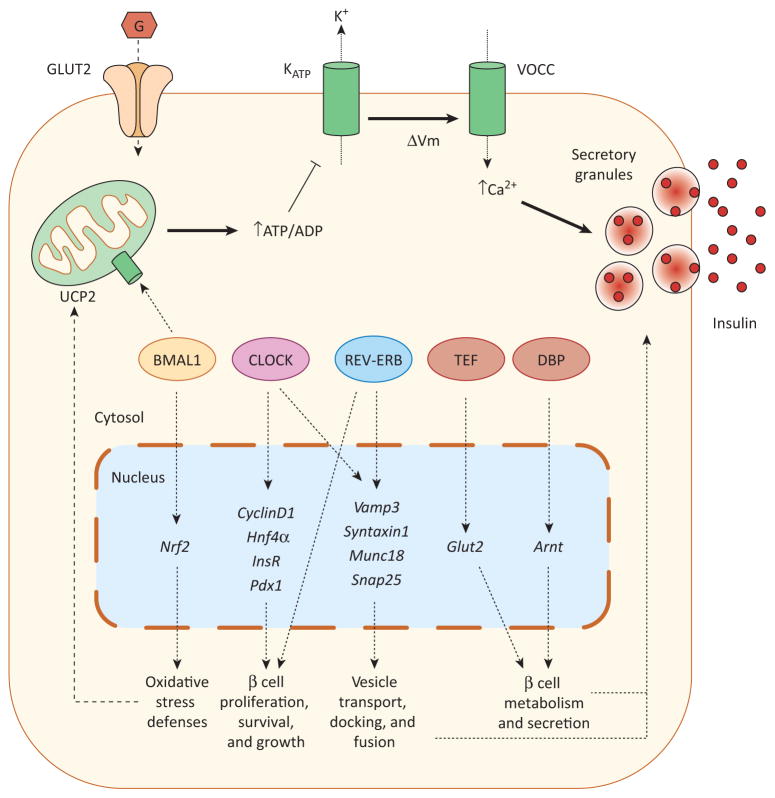
Clock genes in the regulation of pancreatic β cell function. Glucose enters the pancreatic β cell via glucose transporter 2 (GLUT2) and is metabolized by mitochondria, resulting in the rapid generation of ATP and the subsequent closure of ATP-sensitive K+ (KATP) channels. The closure of these channels leads to plasma membrane depolarization (ΔVm), opening voltage-gated Ca2+ channels (VOCC). The consequent Ca2+ influx triggers insulin release through exocytosis of insulin secretory granules. Pancreatic β cell regulation by clock genes may involve several processes and mechanisms. Impaired glucose-induced insulin secretion (GSIS) has been shown in islets deficient in CLOCK, BMAL1, or REV-ERB. CLOCK and REV-ERB may regulate insulin secretion by modulating the expression of genes involved in exocytosis (Vamp3, Syntaxin1, Munc18, Snap25). Additionally, CLOCK and REV-ERB regulate β cell proliferation, probably through the modulation of different genes involved in β cell survival and growth (CyclinD1, Hnf4α, InsR, Pdx1). BMAL1 is also required for normal β cell GSIS by reducing UCP2-mediated mitochondrial uncoupling and oxidative stress and by increasing antioxidant defenses. This latter action is mediated by direct transcriptional modulation of Nrf2 by BMAL1. The clock-controlled output proteins TEF and DBP have also been shown to directly modulate the transcriptional activity of the glucose transporter 2 (Glut2) and Arnt, respectively. This latter gene plays a key role in β cell metabolism and GSIS. Abbreviations: BMAL1, brain and muscle ARNT-like 1; CLOCK, circadian locomotor output cycles protein kaput; CRY, cryptochrome; DBP, d-binding protein; PER, period homolog Drosophila; REV-ERB ALPHA, reverse-eritroblastosis virus α; TEF, thyrotroph embryonic factor; UCP2, uncoupling protein 2.