Fig. 4.
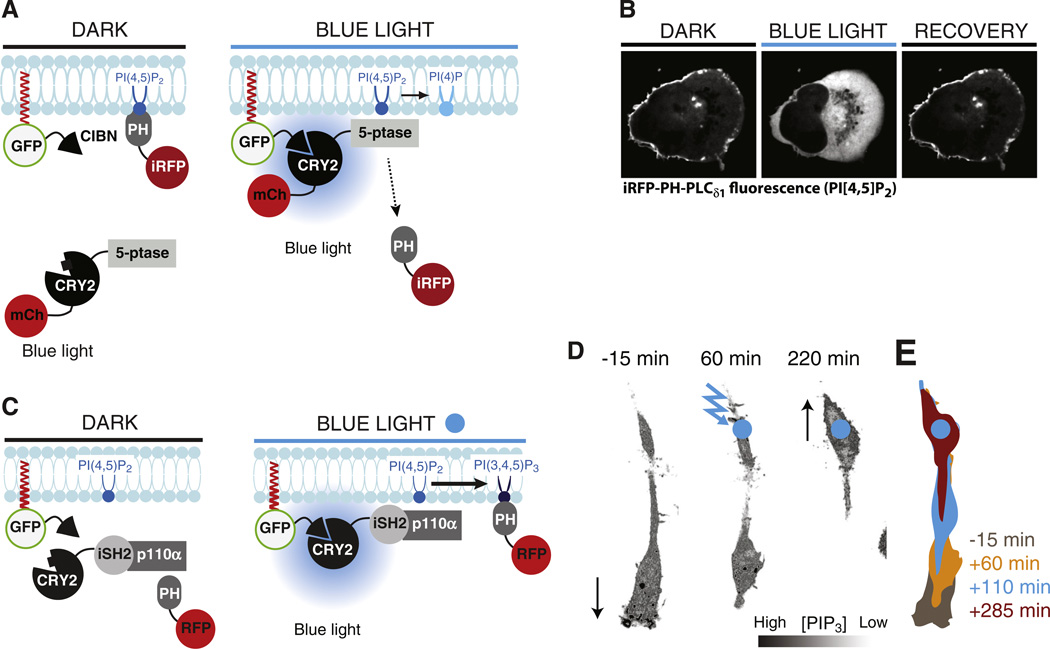
Manipulation of PIs by the blue-light-induced CRY2-CIBN heterodimerization system. A. Schematic drawing showing the principle underlying blue-light-induced PI(4,5)P2 dephosphorylation. CIBN is targeted to the plasma membrane while CRY2 is fused to a 5-phosphatase (5-ptase) domain and carries an mCherry-tag (mCh) for microscopic detection. mCh-CRY2-5-ptase is cytosolic under dark conditions but binds CIBN, and thus is recruited at the target membrane, upon blue-light illumination. This translocation results in dephosphorylation of PI(4,5)P2 and this reaction can be detected as loss of iRFP-PH-PLCδ1 [a PI(4,5)P2 binding module] from the plasma membrane. B. Confocal microscopy images of a HeLa cell showing loss of iRFP-PH-PLCδ1 from the plasma membrane upon blue-light-induced recruitment of mCh-CRY2-5-ptase to this cellular compartment. Pictures were taken 10 s before, 10 s after and 15 min after a 200 ms pulse of blue-light illumination [112]. C. Schematic drawing showing the principle underlying blue-light-induced PI(3,4,5)P3 synthesis via the recruitment of the endogenous p110α subunit of type I PI 3-kinase to a CRY2 fusion of the iSH2-region of its regulatory subunit p85 [101,112]. PI(3,4,5)P3 is detected by the RFP-tagged PH-domain from Akt. D. Confocal microscopy images of a migrating RAW264.7 macrophage expressing the fusion proteins shown in C. Continuous focal illumination (blue circle) results in local PI(3,4,5)P3 synthesis and reverses the direction of cell movement occurring before illumination by inducing local ruffling (arrows). E. Illustrations showing the changes in cell shape and position induced by local PI(3,4,5)P3 synthesis.