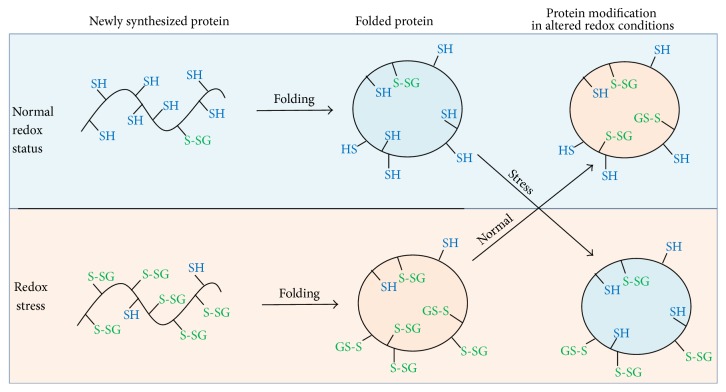
Figure 7.
Schematic representation of Na,K-ATPase glutathionylation depending on intracellular redox status. At normal redox status, the level of GSH is about 100 times higher than GSSG. Under these conditions, during biosynthesis, the proteins are slightly glutathionylated. Redox stress leads to the shift in GSH/GSSG ratio that induces protein glutathionylation. At normal redox status and at redox stress, the basal levels of glutathionylation (glutathionylation of solvent-inaccessible cysteine residues) are different. Subsequent change in the redox status does not affect basal glutathionylation, which demonstrates that the protein “memorizes” a cellular redox state during its biosynthesis. In contrast, glutathionylation of the solvent-accessible cysteine residues (regulatory glutathionylation) depends on the current redox status of cell.