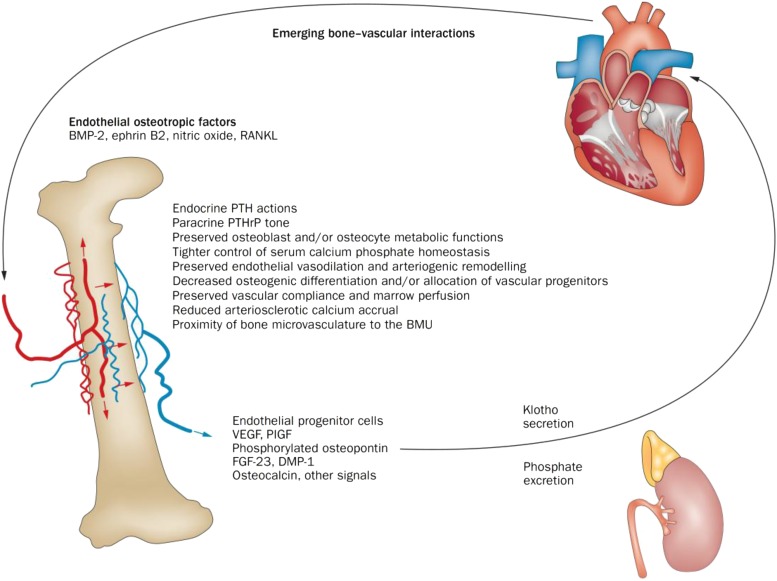
FIGURE 1.
Emerging bone-vascular interactions. A bidirectional endocrine relation exists between bone and the vasculature that mutually benefits bone and vascular health. The kidney is an important intermediary in this process via regulation of phosphate excretion (57) and expression of Klotho (58, 59). Importantly, PTH1R signaling maintains bone formation, sustains hematopoietic niche function (60) and endothelial progenitor cell mass (61), promotes intact osteoblast osteopontin 24 and osteocyte FGF23 (58, 62) secretion, supports renal Klotho production (58), and suppresses aortic osteofibrogenic Wnt/β-catenin signaling (40, 48) and vascular calcium accrual (48, 49). PTH1R signaling also reduces aortic (48) and skeletal (63) oxidative stress and maintains the proximity of the microvasculature to the BMU during bone formation (64). Declining renal function and tissue resistance to PTH1R signaling are key features in the perturbation of the bone-vascular axis in the setting of disease. Age-related changes in marrow composition and the vector of bone perfusion may also functionally perturb the bone-vascular axis. In addition, emerging data point to the role of circulating microvesicles—arising from endothelial cells, smooth muscle cells, and formed elements including platelets—in the endocrine regulation of bone-vascular interactions. BMP, bone morphogenic protein; BMU, basic multicellular unit; DMP-1, dentin matrix protein-1; FGF23, fibroblast growth factor 23; PlGF, placental growth factor; PTH, parathyroid hormone; PTHrP, parathyroid hormone related peptide; PTH1R, PTH/PTHrP receptor; RANKL, receptor activator of nuclear factor κB ligand; VEGF, vascular endothelial growth factor; Wnt, wingless-type mouse mammary tumor virus integration site family member. Reproduced from reference 6 with permission.