Figure 3. Opposite effects of NF-κB modulators on HIV-1 uDNA and iDNA transcriptional activities.
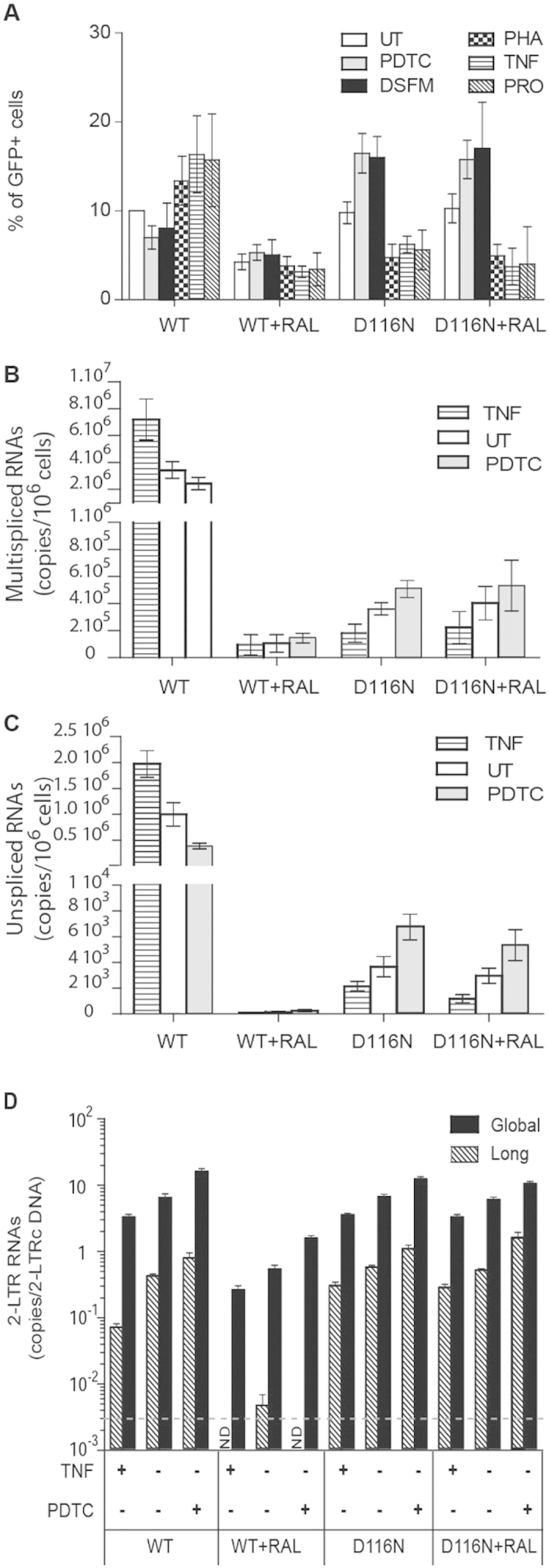
(A) Impact of pharmacological treatments modulating the NF-κB pathway on HIV reporter gene expression depending on the viral integrative status. MT4 T-cells were infected with HIV-1 env−gfp+ harboring either wild-type (WT) integrase or a catalytically defective (“D116N”) integrase, each pseudotyped with VSV-G protein (4 ng of p24gag antigen per 106 cells; ≈m.o.i. 0.1). When indicated, 200 nM RAL was added 1 h before infection. Two days later, infected cells were incubated or not (UT) with pharmacological agents inhibiting the NF-κB pathway [50 μM pyrrolidinedithiocarbamate (PDTC), 10 μM disulfiram (DSFM)], or stimulating it [2.5 μg/mL phytohemagglutinin (PHA), 100 ng/mL tumor necrosis factor alpha (TNF), 5 μM prostratin (PRO)]. Three days post-infection, cytofluorometry was employed to determine the percentage of GFP-expressing cells. The results (mean ± confidence intervals for a p value < 0.05), were obtained from 3 to 7 independent experiments depending on the conditions. Treatments were maintained during the time of the experiment. (B–D) Impact of pharmacological treatments modulating the NF-KB pathway on HIV transcription depending on the viral integrative status. MT4 T-cells were treated and infected in the same conditions as described in panel A. Three days later, the amounts of unspliced, multi-spliced, long and global 2-LTR HIV-1 RNAs were determined by RT-qPCR. The efficiency of 2-LTRc transcription measured as the copy number of global or long 2-LTR RNAs per LTRc DNA template is presented in panel D. The grey line represents the detection threshold of long 2-LTR RNAs per 2-LTRc copy. The results (mean ± confidence intervals for a p value < 0.05 in panel B and C, mean ± s.e.m for panel D), were obtained from 3 to 6 independent experiments depending on the conditions. nd = not detected.
