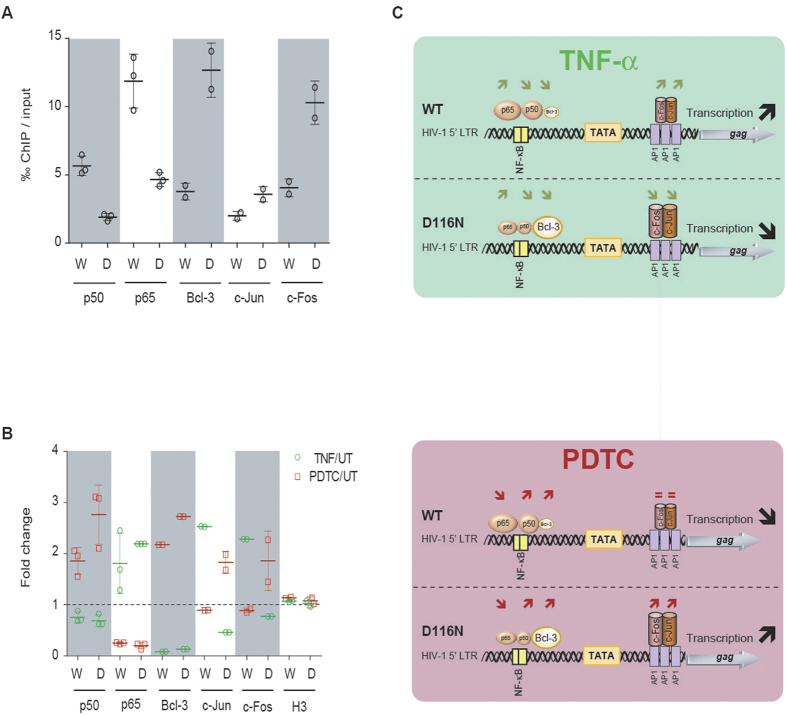
Figure 4. Differential binding of transcription factors to HIV-1 uDNA and iDNA depending on NF-κB pathway modulation.
MT4 T-cells were infected with HIV-1 NL4-3 strains harboring a wild-type (“WT” or “W”) or a catalytically defective (“D116N” or “D”) integrase, each pseudotyped with VSV-G protein (100 ng of p24gag antigen per 106 cells; ≈m.o.i. 1). Two days later, the cells were incubated or not (UT) with pharmacological agents stimulating [100 ng/mL tumor necrosis factor alpha (TNF)] or inhibiting [50 μM pyrrolidinedithiocarbamate (PDTC)] the NF-κB pathway. Chromatin immunoprecipitation (ChIP) assays were performed on cellular extracts harvested 3 days post-infection, with antibodies directed against p50/NF-κB, p65/NF-κB, Bcl-3, c-Jun, c-Fos and H3 proteins. DNA obtained from the input or from the immunoprecipitated DNA were applied to qPCR performed on total HIV-1 DNA. The proportion of immunoprecipitated material per thousand (‰) of input material (normalized on immunoprecipitation yield) is presented in the panel A for p50, p65, Bcl-3, c-Jun and c-Fos ChIP assays. ChiP experiments performed with H3 antibodies led to the immunoprecipitation of 32–57% and 0.6–13% of input material in WT and D116N conditions, respectively. In panel (B), the results are expressed as the relative fold change of immunoprecipitated material in TNF-α or PDTC treated conditions compared to untreated condition. Schematic representations of ChIP and transcription results are presented in panel (C). Differences in the amount of proteins bound to WT vs D116N DNA (‰ of input) are represented by relatively smaller or bigger complexes, whereas significant variations after treatment (fold change) are indicated by arrows. The results were obtained from two independent experiments for Bcl-3, c-Jun and c-Fos and 3 independent experiments for NF-κB p65/p50 subunits.