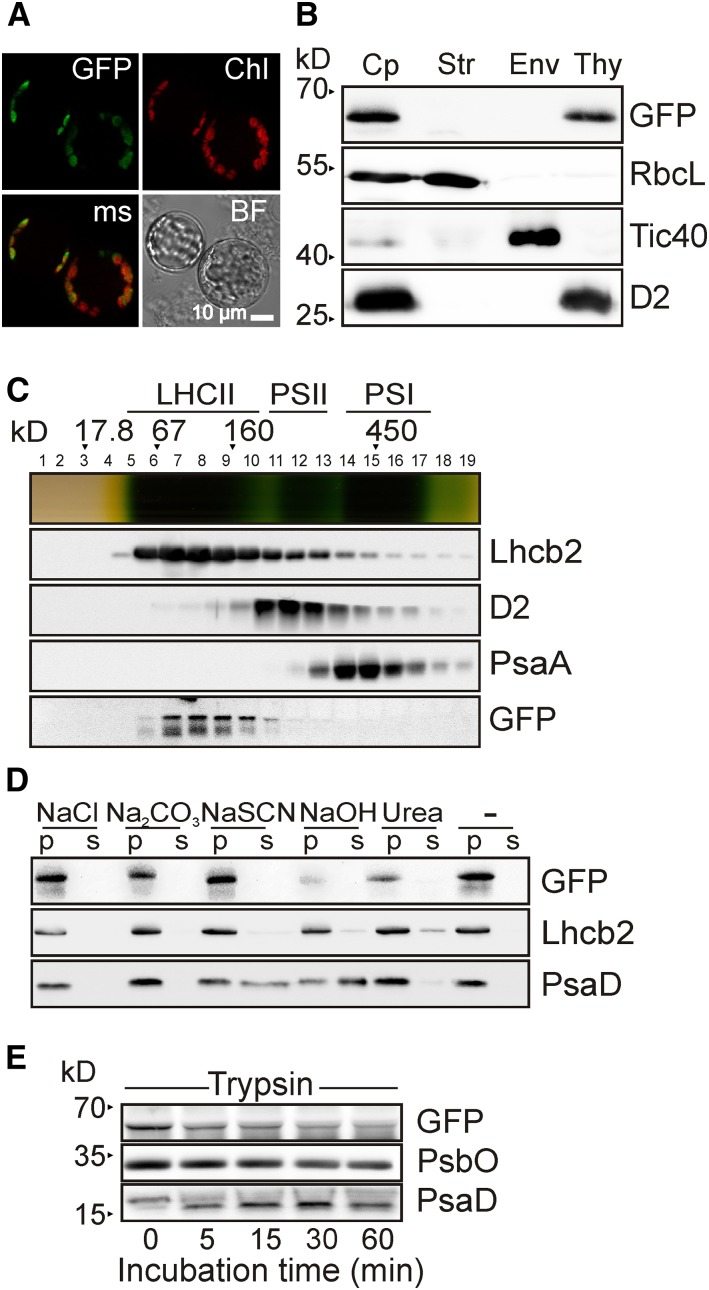
Figure 5.
Thylakoid Localization and Topology of PAM71.
(A) Subcellular localization of PAM71-GFP. Protoplasts were isolated from pam71-1Pro35S:PAM71-GFP, and the fusion protein was localized by fluorescence microscopy. GFP fluorescence was excited at 470 ± 40 nm, and the emission was recorded at 525 ± 50 nm. Chlorophyll autofluorescence (Chl) was excited at 450 to 490 nm, and emission was recorded at >515 nm. The obtained signals were merged (ms); a bright-field (BF) photograph showed intactness of the protoplasts.
(B) Suborganellar localization of PAM71-GFP. Total chloroplasts (Cp) were isolated from pam71-1Pro35S:PAM71-GFP and fractionated into stroma (Str), envelope membranes (Env), and thylakoid membranes (Thy). Immunodetection was performed with anti-RbcL (a marker for the stroma fraction), anti-Tic40 (envelope membrane fraction), anti-D2 (thylakoid membrane fraction), and anti-GFP to detect PAM71-GFP.
(C) Thylakoid membrane fractionation of PAM71-GFP. Thylakoids (1 mg chlorophyll mL−1) of pam71-1Pro35S:PAM71-GFP were solubilized with 1% (w/v) β-dodecyl maltoside, and complexes were separated in a linear 0.1 to 1 M sucrose gradient. Nineteen fractions were collected, proteins from each fraction were precipitated, and immunoblot analysis was performed using anti-Lhcb2, anti-D2, anti-PsaA, and anti-GFP antibodies. The positions of molecular mass markers and of the major complexes (LHCII, PSII, and PSI) are indicated.
(D) PAM71-GFP is an integral membrane protein. Thylakoid membranes were isolated from pam71-1Pro35S:PAM71-GFP and exposed to chaotropic salts or alkaline pH. Membranes were resuspended at 0.5 mg chlorophyll mL−1 in 10 mM HEPES (pH 7.5) containing either 2 M NaCl, 0.1 M Na2CO3, 2 M NaSCN, 0.1 M NaOH, 6 M urea, or no additive. After incubation for 30 min on ice, supernatants containing soluble proteins (S) or pelleted proteins (P) were fractionated by SDS-PAGE, and immunoblot analysis was performed using anti-GFP, anti-Lhcb2, and anti-PsaD antibodies. Note that PAM71-GFP behaves like the integral membrane protein Lhcb2.
(E) Membrane accessibility of PAM71-GFP. Isolated thylakoid membranes from pam71-1Pro35S:PAM71-GFP were treated with trypsin for the indicated times. Immunoblot analysis was performed using anti-GFP, anti-PsbO, and anti-PsaD antibodies. Note that only ∼2 kD of PsaD is susceptible to proteolysis, in agreement with observations by Minai et al. (1996).