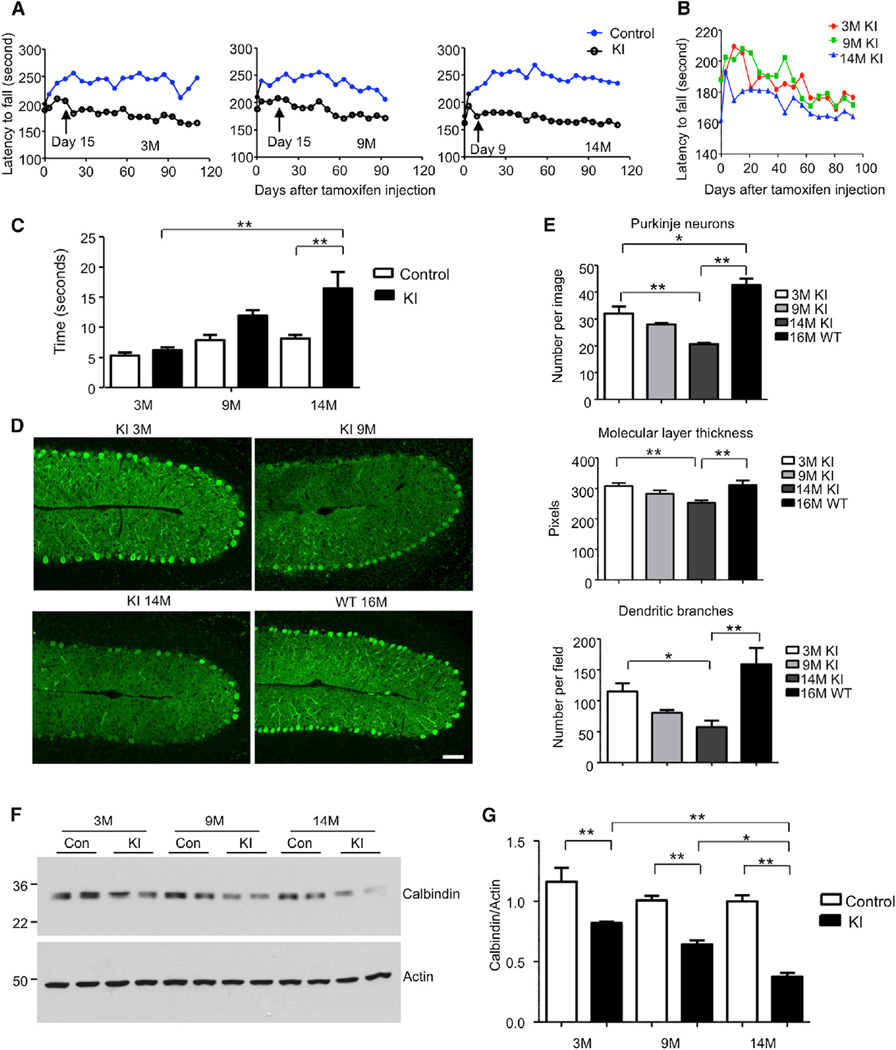
Figure 2. Aging Exacerbates Neurological Phenotypes in TBP105Q Inducible KI Mouse Model.
(A) Motor activity of mice injected with tamoxifen at 3, 9, and 14 months of age was analyzed by rotarod test. The older KI mice (day 9 for 14 months, p = 0.0031) showed earlier on set of motor impairment than the younger KI mice (day 15 for 9 months, p = 0.0298, and day 15 for 3 months, p = 0.0217) after tamoxifen injection (n = 9–11 each group, two-way ANOVA, p < 0.0001).
(B) The rotarod performance of differently aged KI mice was also compared.
(C) Motor coordination of mice was tested by balance beam walking assay 1.5 months after tamoxifen injection. The walking time of older TBP105Q inducible KI mice on the beam is significantly longer than that of young KI mice (n = 6; **p < 0.01).
(D) Degeneration of Purkinje neurons labeled by anticalbindin in the cerebellum of KI mice injected with tamoxifen at different ages. WT mouse at 16 months of age served as a control. The scale bar represents 50 µm.
(E) Quantification of Purkinje neuron number, molecular layer thickness, and dendritic branches in TBP105Q inducible KI mice at different ages (n = 6–8; *p < 0.05, **p < 0.01).
(F and G) Western blotting (F) and quantitative analysis (G) of calbindin protein level in the cerebellum of 3-, 9-, and 14-month-old TBP105Q inducible KI mice. Actin was used as a loading control (*p < 0.05, **p < 0.01). Data are represented as mean ± SEM.