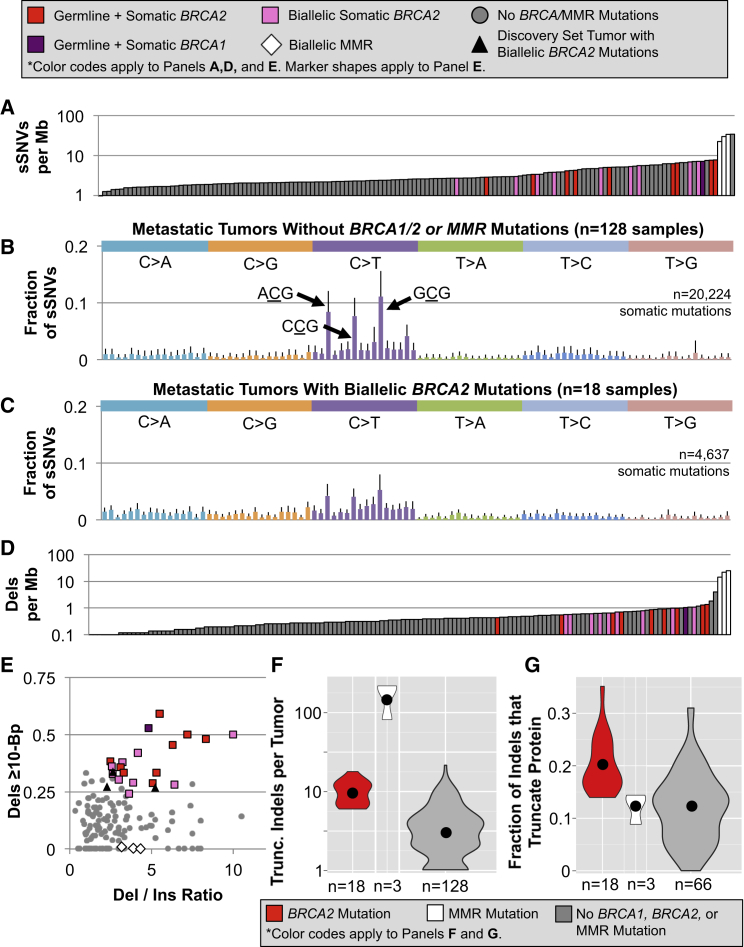
Figure 4.
Nearly Identical Somatic Mutation Signatures Were Detected in Our BRCA2 Deficiency-Targeted Reanalysis of 150 Metastatic Prostate Tumors,8 18 with Biallelic Loss of BRCA2, 1 with Biallelic Mutations in BRCA1, and 3 with Biallelic MMR Defects
(A) Germline and somatic defects shared a statistically indistinguishable propensity to cause a modest increase in the sSNV mutation rate.
(B) C>T substitutions were most frequent in metastatic HR-competent PCa, exhibiting even greater enrichment than observed in the discovery set tumors.
(C) In contrast, C>T transitions were not dramatically overrepresented in the BRCA2-deficient tumors. Much of the difference is derived from a paucity of substitutions within the triplets ACG, CCG, and GCG, which were characteristic in their HR-intact counterparts.
(D) As seen in these tumors among sSNVs, germline and somatic HR defects led to a similar rise in the indel mutation rate.
(E) Samples with biallelic HR deficiency had enrichment for both deletions over insertions and long deletions, recapitulating the trend observed in the discovery set tumors.
(F) The number of truncating indels was partially determined by differences in the somatic indel rate.
(G) BRCA2-mutated tumors had a higher percentage of indels that were predicted to truncate the encoded protein.
For all panels with error bars, these represent the standard deviation from the mean of all samples in that category.