Figure 2.
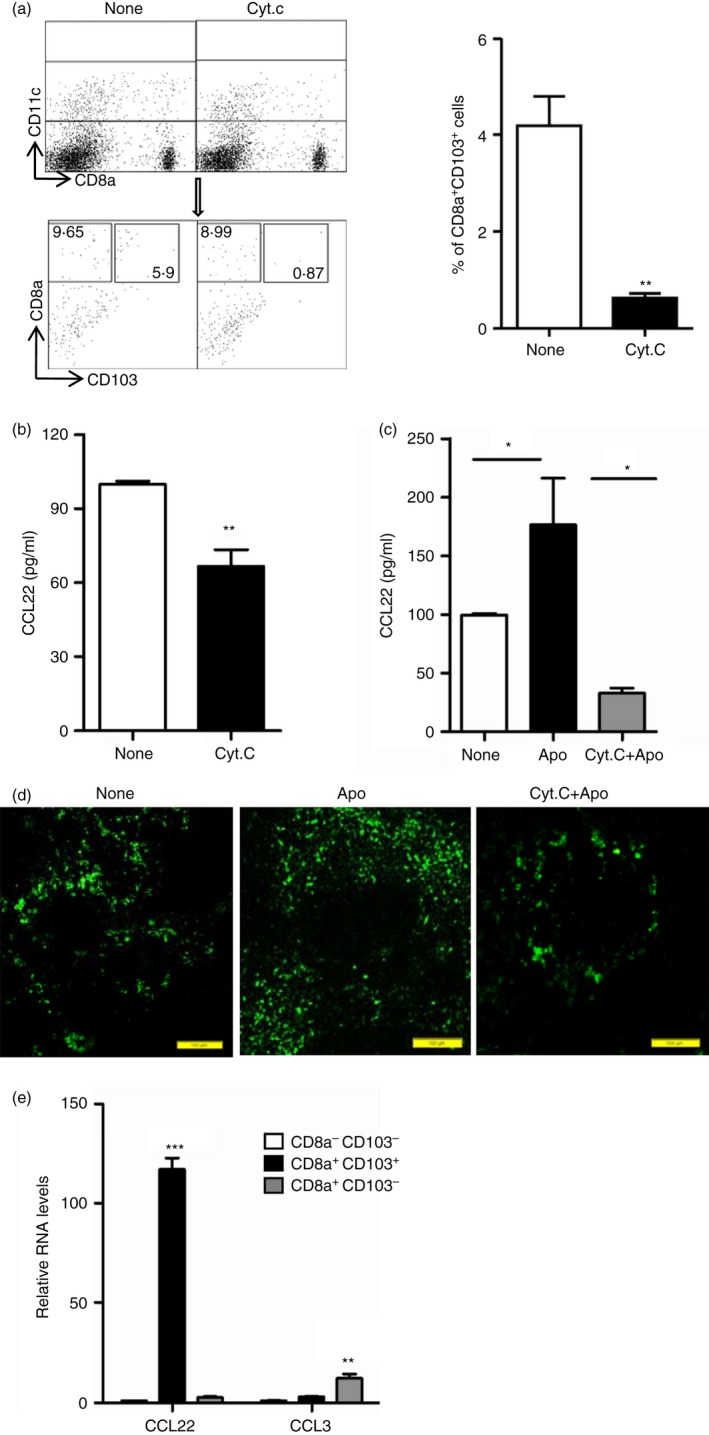
Splenic CD8α + CD103+ dendritic cells (DCs) dominantly secrete CCL22. (a) C57BL/6J mice were injected intravenously with 5 mg of Cytochrome c (CytC) or not (None). Twenty‐four hours later, splenocytes were isolated and stained with antibodies for CD11c, CD8α and CD103 for flow cytometry analysis, and the percentages of CD8α + CD103+ DCs in CD11c+ gated cells were compared. (b) Splenic CD11c+ DCs enriched from mice treated as described in (a) by MACS,were cultured in vitro for CCL22 production detection using ELISA. (c, d) Cytochrome c was injected intravenously into mice. Twenty‐four hours later, the mice were injected intravenously with 1 × 107 apoptotic thymocytes. Twelve hours later, splenic CD11c+ DCs were enriched by MACS from control (None), apoptotic cell injection only (Apo) and CytC with apoptotic‐cell‐injected (CytC+Apo) mice and cultured for CCL22 production detection using ELISA (c) and spleens from these three groups of mice were used for immunohistochemistry analysis of CCL22 expression (d). (e) Splenocytes from control mice (None) and apoptotic‐cell‐challenged mice (Apo) were stained with antibodies for CD11c, CD8α and CD103, and CD8α − CD103− DCs, CD8α + CD103− DCs and CD8α + CD103+ DCs in CD11c+ gated cells were sorted and used for examining the mRNA expression level of CCL22 and CCL3. *P < 0·05, **P < 0·01,***P < 0·001 compared with control, respectively.
