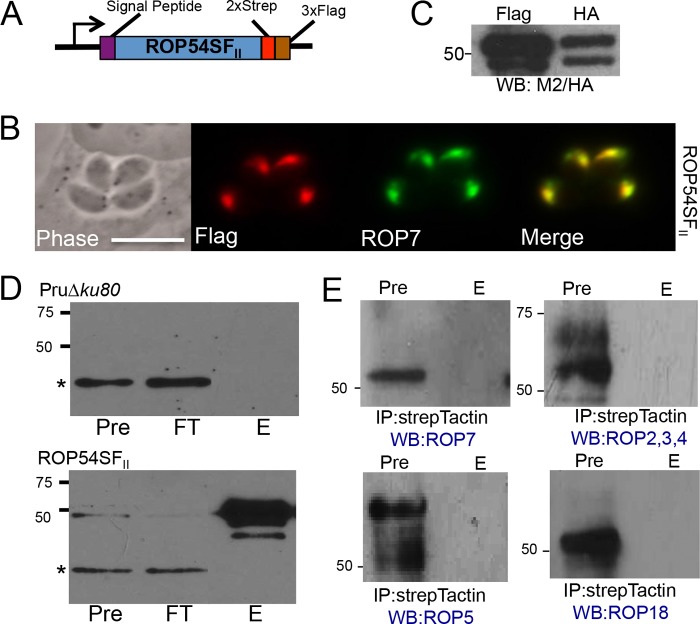
FIG 3 .
Purification of ROP54 indicated that there is no robust interaction with other known ROP effector proteins. (A) Illustration showing the endogenously tagged ROP54 with predicted signal peptide, coding region, and C-terminal 2×Strep 3×Flag epitope tags. (B) IFA with anti-Flag antibody showed colocalization with the rhoptry protein ROP7. (C) Western blot assay results for ROP54SFII and ROP54HAII parasite lines demonstrated that the slower-migrating band was the main band of ROP54. (D) Western blotting results with precolumn (Pre), flowthrough (FT), and elution (E) fractions of the Pru∆ku80 (top) and ROP54SF (bottom) StrepTactin pulldown product probed with mouse anti-Flag antibody. A nonspecific band is represented by the asterisk. (E) IP-Western blot probing for known ROP kinases and pseudokinase after ROP54SF pulldown.