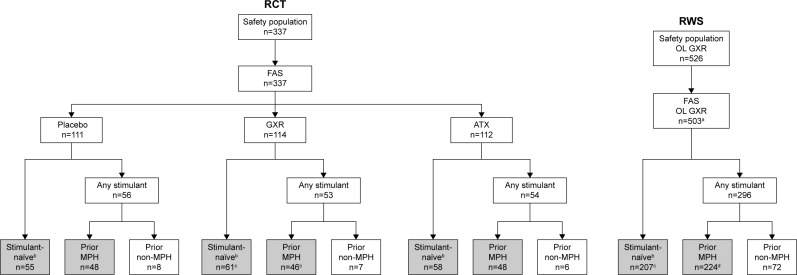
Figure 1.
Participant disposition in the randomized controlled trial and open-label phase of the randomized-withdrawal study.
Notes: aExcludes participants from site 801 (due to breach of good clinical practice that was reported to applicable authorities). bIncludes participants who were treatment-naïve or who reported prior use of non-stimulant ADHD medications. cOne participant was excluded from the efficacy analyses due to no available post-baseline measurements. dThree participants were excluded from the ADHD-RS-IV efficacy analyses, and two participants were excluded from the CGI-I efficacy analyses due to no available post-baseline measurements. Based on responses in the Prior Stimulant Medication Questionnaire, participants were categorized as either stimulant-naïve or having received any prior stimulant. Those who had received stimulants were grouped by the type of the most recent stimulant (ie, last stimulant received prior to the 3- to 35-day washout/screening period). The efficacies of GXR and ATX versus placebo were compared in participants who had received prior MPH and stimulant-naïve (gray shading).
Abbreviations: ADHD, attention-deficit/hyperactivity disorder; ADHD-RS-IV, ADHD Rating Scale version IV; ATX, atomoxetine; CGI-I, Clinical Global Impression – Improvement scale; FAS, full-analysis set; GXR, guanfacine extended release; MPH, methylphenidate; OL, open-label; RCT, randomized controlled trial; RWS, randomized-withdrawal study.