Abstract
Background
Duchenne muscular dystrophy (DMD) is characterized by progressive skeletal muscle and cardiac dysfunction. While skeletal muscle dysfunction precedes cardiomyopathy, the relationship between the progressive decline in skeletal and cardiac muscle function is unclear. This relationship is especially important given that the myocardial effects of many developing DMD therapies are largely unknown.
Objective
Our objective was to assess the relationship between progression of skeletal muscle weakness and onset of cardiac dysfunction in DMD.
Methods
A total of 77 DMD subjects treated at a single referral center were included. Demographic information, quantitative muscle testing (QMT), subjective muscle strength, cardiac function, and current and retrospective medications were collected. A Spearman rank correlation was used to evaluate for an association between subjective strength and fractional shortening. The effects of total QMT and arm QMT on fractional shortening were examined in generalized least square with and without adjustments for age, ambulatory status, and duration of corticosteroids and cardiac specific medications.
Results
We found a significant correlation between maintained subjective skeletal muscle arm and leg strength and maintained cardiac function as defined by fractional shortening (rho=0.47, p=0.004 and rho=0.48, p=0.003, respectively). We also found a significant association between QMT and fractional shortening among non-ambulatory DMD subjects (p=0.03), while this association was not significant in ambulatory subjects.
Conclusions
Our findings allow us to conclude that in this population, there exists a significant relationship between skeletal muscle and cardiac function in non-ambulatory DMD patients. While this does not imply a causal relationship, a possible association between skeletal and cardiac muscle function suggests that researchers should carefully monitor cardiac function, even when the primary outcome measures are not cardiac in nature.
Keywords: Duchenne muscular dystrophy, Cardiac dysfunction, Cardiomyopathy, Skeletal muscle dysfunction, Ambulation
1. INTRODUCTION
Duchene Muscular dystrophy (DMD) is an X-linked disease that occurs in 1 in 4700 male births.(1) It results from a mutation in the gene for dystrophin that leads to progressive skeletal muscle and cardiac dysfunction.(2) The majority of patients experience loss of ambulation between 10–13 years of age and develop cardiac dysfunction later during their teenage years.(3, 4) Medical therapies, including corticosteroids and non-invasive ventilation, have extended the life expectancy of DMD patients into the fourth decade.(5) As a result of this increased lifespan, cardiovascular disease has become the leading cause of DMD mortality.(6)
While the pathogenesis of muscle dysfunction in DMD is not completely understood, dystrophin is part of the dystrophin glycoprotein complex. Loss of dystrophin leads to dysregulation of calcium homeostasis. This results in muscle cell injury and death, inflammation, fibrosis, and fibro-fatty replacement.(7) These processes are presumed to be similar in skeletal and cardiac muscle. Despite this, skeletal muscle dysfunction precedes left ventricular (LV) dysfunction and it is unclear how these relate. Multiple authors have postulated that these processes are unrelated.(8) With the development of new DMD therapeutic options, many of which increase skeletal muscle strength but have unknown myocardial effects, understanding this relationship has become more important.(9)
It has been postulated that improved skeletal muscle strength in DMD leads to an increase in myocardial stress and earlier cardiac dysfunction.(10) This model is based on patients with Becker muscular dystrophy (BMD) who can present with minimal skeletal weakness and severe cardiac dysfunction. While animal models have led to conflicting results, the only human data suggest a linear relationship between skeletal and cardiac dysfunction in ambulatory boys that refutes the BMD hypothesis.(11) The objective of this study was to evaluate skeletal muscle and cardiac function in a cohort of DMD boys and examine the relationship between progression of skeletal muscle weakness and onset of cardiac dysfunction in both ambulatory and non-ambulatory boys with DMD.
2. MATERIALS AND METHODS
2.1 Participants
The Vanderbilt Institutional Review Board approved this study. The electronic medical record was reviewed in order to identify subjects evaluated with DMD between 1995–2013. Inclusion criteria were the following: 1) all identifiable subjects with DMD evaluated at Vanderbilt during the study time period, 2) diagnosis of DMD confirmed with clinical phenotype and either muscle biopsy or genetic testing. Exclusion criteria were the following: 1) Unclear neuromuscular diagnosis or diagnosis of other neuromuscular disease, 2) No echocardiogram performed or no objective measures of LV function that could be paired with measures of skeletal muscle function. A sample of 86 DMD subjects born between 1977–2009 was identified; 77 subjects were included.
Demographics, current and previous medications, and past medical history were collected from the electronic medical record. Medications recorded included corticosteroids, angiotensin converting enzyme inhibitors, beta-blockers, and angiotensin receptor blockers.
All previous echocardiograms were reviewed. Cardiac function data was collected, including fractional shortening (FS) and left ventricular ejection fraction (LVEF). Chamber sizes included the following: the LV internal dimension in systole and diastole, the LV posterior wall dimensions in diastole, the interventricular septal thickness in diastole, and the left atrial dimension. LV function was defined as abnormal if FS was less than 28% or LVEF was less than 55%. FS was used as primary analysis for the purpose of this study as it was measured in the majority of studies and has been shown to be both reproducible and to correlate reasonably well with LVEF measured by cardiac MRI.(12, 13) RV function was not analyzed.
All previous neurology visits were reviewed. Ambulatory status was recorded for each visit. Global skeletal muscle assessment data were collected in the form of subjective assessment of arm and leg strength, using a scale of 0 (no movement) to 5 (normal strength), including values of 4 minus and 4 plus. Skeletal muscle assessment was also assessed in the form of quantitative muscle testing (QMT) using a handheld myometer, which is an objective, reproducible method for both upper and lower extremity strength evaluation in DMD.(14, 15) Arm QMT score was calculated by adding flexion and extension values for the right and left elbows. Leg QMT score was calculated by adding flexion and extension values for the right and left knees. Total QMT score was calculated by adding the total arm score and the total leg score.
All neurology visits included skeletal muscle assessment data, either in the form of subjective assessment or QMT. Visits rarely included both types of assessment; subjects were more likely to have undergone subjective assessments at earlier visits and QMT at later visits.
Patients with DMD in our institution are typically evaluated by pediatric neurology every 6 months after diagnosis. The initial cardiology evaluation routinely occurs around 6 years of age. Patients continue to follow annually with cardiology until 10 years of age or the onset of cardiac abnormalities, such as LV dilation, LV dysfunction, or symptoms of heart failure or arrhythmias, at which point patients are followed as needed. Patients are routinely started on cardiac specific medications either with the onset of abnormalities by echocardiography or cardiac MRI or prophylactically at 10 years of age, though the rare patient has either not tolerated or refused therapy. The frequency of neurology and cardiac evaluations is in accordance with published care guidelines.(16, 17) The cardiology and neurology appointments occur on the same day whenever possible. The last cardiac and skeletal muscle assessments were used to analyze current function for demographics. FS and subjective skeletal muscle assessments that occurred within 6 months of each other were paired and included in the analysis. Due to periods without outpatient follow up, poor echocardiographic windows, or incomplete records, not all data points could be paired. Data that could not be paired were excluded from the analysis.
2.4 Statistical analysis
Descriptive statistics are presented as frequencies and proportion (%) for categorical variables, and mean with standard deviation and range for continuous variables. Subjective muscle arm and leg strength testing was paired with a FS measure from an echocardiogram performed within 6 months of the strength testing. Subjective strength testing and FS were performed a median of 9 days apart. The correlation of the most recent of these measures was assessed using Spearman rank correlation test.
QMT was paired with a FS measure from an echocardiogram performed within 4 weeks of the QMT. QMT and FS were performed a median of 0 days apart. The effects of total QMT and arm QMT on FS were examined separately in generalized least square with and without adjustments for age, ambulatory status, and duration of corticosteroids and cardiac specific medications. To avoid over-fitting, only those risk factors deemed most important were pre-specified. Assessment of whether a subject’s ambulatory status modified the effects of QMT on FS levels were conducted by including the cross product of QMT and ambulatory status while controlling for age, ambulatory status, duration of corticosteroids, and duration of cardiac specific medications. In order to prevent overfitting of the model, duration of cardiac specific medications was defined as the number of years on any of the following medications, either alone or in combination: angiotensin converting enzyme inhibitors, angiotensin receptor blockers, or beta-blockers. We evaluated the model assumption through checking distributions of model residuals. Statistical analysis was performed using R studio 3.0.2 (online at http://www.rstudio.com/). All tests were 2-sided and a p-value of less than 0.05 was considered significant. Study data were collected and managed using REDCap (Research Electronic Data Capture) electronic data capture tools hosted at Vanderbilt.(18)
3. RESULTS
3.1 Demographics
At the time of analysis, the average age of the 77 DMD subjects was 14.1 years (range 3–35) (Table 1; of note, all tables reflect demographic data at time of analysis unless otherwise specified); 6 subjects were deceased at the time of data collection. There were 48 subjects (62%) who were non-ambulatory with loss of ambulation occurring at a mean age of 10.6 ± 2.0 years. There were 25 subjects (33%) who had developed LV systolic dysfunction at a mean age of 15.4 ± 4.7 years (range 8–27). Of those subjects with LV systolic dysfunction, 21 of 25 subjects (84%) were non-ambulatory prior to developing dysfunction. Two of these subjects had low-normal function on their most recent echocardiograms.
Table 1.
Baseline characteristics of study patients (at time of analysis)
| Characteristics | N = 77 |
|---|---|
| Male | 77 (100%) |
|
| |
| Current age (years) | 14.1 ± 6.4 (range 3–35) |
|
| |
| Current number of non-ambulatory subjects | 48 (62%) |
| Mean age of loss of ambulation | 10.6 ± 2.0 (range 7–16) |
|
| |
| Current subjects with LV dysfunction | 25 (33%) |
| Mean age of onset of LV dysfunction | 15.4 ± 4.7 (range 8–27) |
|
| |
| Medications | |
|
| |
| Current or previous corticosteroids | 78% (N=60) |
| Duration corticosteroids (years) | 3.4 ± 2.5 |
|
| |
| Current or previous ACEi* | 48% (N=37) |
| Duration ACEi (years) | 2.9 ± 2.9 |
|
| |
| Current or previous ARB† | 12% (N=9) |
| Duration ARB (years) | 3.9 ± 1.8 |
|
| |
| Current or previous beta blocker | 30% (N=23) |
| Duration beta blocker (years) | 3.3 ± 2.0 |
|
| |
| Current or previous mineralocorticoid | 4% (N=3) |
| Duration of mineralocorticoid (years) | 2 ± 1.8 |
|
| |
| Current skeletal muscle strength | |
| Median subjective arm strength | 4 |
| Median subjective leg strength | 4 minus |
| Mean QMT‡ (pounds) | 73.1 ± 39.6 |
Angiotensin converting enzyme inhibitor (ACEi)
Angiotensin receptor blocker (ARB)
Quantitative muscle testing (QMT)
As expected, non-ambulatory subjects were older than ambulatory subjects (17.5 years vs 8.5 years, p<0.001) (Table 2). A higher percentage of non-ambulatory subjects had LV dysfunction (46% vs 10%).
Table 2.
Clinical features of ambulatory and non-ambulatory DMD subjects (at time of analysis)
| Characteristics | Non-ambulatory N=48 |
Ambulatory N=29 |
p-value |
|---|---|---|---|
| Current age (years) | 17.5 ± 5.5 | 8.5 ± 3.1 | <0.001* |
| Age at loss of ambulation (years) | 10.6 ± 2.0 | ||
|
| |||
| Current subjects with LV dysfunction | 46% (N=22) | 10% (N=3) | 0.001† |
| Age LV dysfunction | 16.3 ± 4.2 | 9.0 ± 1.2 | 0.001* |
| Current mean fractional shortening (%) | 28.5 ± 8.6 | 34.0 ± 7.0 | 0.002* |
| Range | (4.7%–40%) | (11.7%–35%) | |
|
| |||
| Medications | |||
|
| |||
| Current or previous corticosteroids | 73% (N=35) | 86% (N=25) | 0.173† |
| Duration corticosteroids (years) | 3.6 ± 2.8 | 3.2 ± 2.1 | 0.898* |
|
| |||
| Current or previous ACEi‡ | 58% (N=28) | 31% (N=9) | 0.020† |
| Duration ACEi (years) | 3.6 ± 3.0 | 0.9 ± 0.9 | 0.001* |
|
| |||
| Current or previous ARB§ | 19% (N=9) | 0 | 0.013† |
| Duration ARB (years) | 3.9 ± 1.8 | ||
|
| |||
| Current or previous ACEi or ARB | 69% (N=33) | 31% (N=9) | 0.001† |
| Duration ACEi + ARB (years) | 4.1 ± 2.8 | 0.9 ± 0.9 | <0.001* |
|
| |||
| Current or previous Beta blocker | 42% (N=20) | 10% (N=3) | 0.004† |
| Duration beta blocker (years) | 3.6 ± 1.9 | 1.3 ± 1.5 | 0.076* |
| Current or previous mineralocorticoid | 4% (N=2) | 3% (N=1) | 0.684‖ |
| Duration mineralocorticoid (years) | 2.8 ± 1.8 | 0.5 | |
Mann-Whitney U test
Chi-Square test
Angiotensin converting enzyme inhibitor (ACEi)
Angiotensin receptor blocker (ARB)
Fisher’s exact test
3.2 Medical Therapy
Sixty subjects (78%) were currently treated with or had been treated with corticosteroids, with a mean duration of therapy of 2.7 ± 2.6 years (range 0–9 years). Forty-seven subjects (61%) were receiving or had been treated with at least one cardiac specific medication (angiotensin converting enzyme inhibitors, angiotensin receptor blockers, or beta-blockers). Of the 30 subjects who had not received cardiac medications, 18 of them were under 10 years of age. The most common cardiac specific medications were angiotensin converting enzyme inhibitors, with 48% of cohort either currently or previously taking a medication in that class (N=37). All subjects with LV dysfunction were initially started on at least one cardiac specific medication, 18 with angiotensin converting enzyme inhibitors, 7 with angiotensin receptor blockers, 17 with beta-blockers, and 3 with mineralocorticoids. The dosing and number of cardiac medications changed with the clinical condition. Given the significant number of subjects treated with corticosteroids and cardiac specific medications, these medications were adjusted for in the model.
The total duration of corticosteroids was not significantly different between ambulatory and non-ambulatory subjects; non-ambulatory subjects had a longer duration of angiotensin converting enzyme inhibitor therapy, likely due to their older age (Table 2). Ambulatory patients had an earlier onset of LV dysfunction, but this was likely biased by the small number of subjects with LV dysfunction in this group and the overall younger age of ambulatory subjects. There was no significant difference in total QMT, arm QMT, or leg QMT in patients taking or not taking cardiac specific medications, though the study was underpowered to detect a difference.
3.3 Skeletal Muscle and Cardiac Function
A total of 264 sets of subjective arm and leg strength measurements were performed in 48 subjects. The median subjective arm strength was 4 and the median subjective leg strength was 4 minus. The median subjective arm and leg strength was 4 in ambulatory subjects; in non-ambulatory subjects, the median subjective arm strength was 1 and the median subjective leg strength was 0 (Table 3) (p<0.001 for both). A total of 290 sets of measurements of QMT were performed in 46 subjects (Mean number ~6.3 per subject). The mean QMT was 102.6 ± 37.3 pounds in ambulatory subjects compared with 54.1 ± 28.1 pounds in non-ambulatory subjects (p<0.001). Non-ambulatory subjects had significantly more QMT assessments than ambulatory subjects (Table 4). Subjects with and without LV dysfunction had no significant difference in number of QMT assessments (5.2 ± 3.6 with LV dysfunction vs 6.7 ± 3.1 without, p=0.18). Those with LV dysfunction had lower total, arm, and leg QMT (81.9 ± 38.8 pounds vs 48.2 ± 31.4 pounds p=0.005, 29.8 ± 15.5 pounds vs 18.5 ± 11.4 pounds p=0.029, and 34.1 ± 32.2 pounds vs 14.2 ± 20.7 pounds p=0.009, respectively). A total of 342 echocardiograms were performed, with a mean number of echocardiograms of 4.4 ± 3.4 per subject. The FS correlated moderately with subjective arm strength (rho=0.47, r2=0.22) and subjective leg strength (rho=0.48, r2=0.23) (p=0.004 and p=0.003, respectively).
Table 3.
Clinical features of DMD subjects with and without left ventricular (LV) dysfunction at most recent echocardiogram
| Characteristics | Normal LV function N=54 |
Abnormal LV function N=23 |
p-value |
|---|---|---|---|
| Current age (years) | 11.5 ± 4.7 | 20.2 ± 5.9 | <0.001* |
|
| |||
| Current mean fractional shortening (%) | 35.1 ± 3.4 | 19.3 ± 6.5 | |
| Range | (28.4%–45.2%) | (4.7%–27%) | |
| Current number of non-ambulatory subjects | 26 (48.1%) | N=3 (13%) | 0.004† |
| Current mean QMT (pounds) | 81.9 ± 38.8 | 48.2 ± 31.4 | 0.005* |
|
| |||
| Medications | |||
|
| |||
| Current or previous corticosteroids | 83% (N=45) | 65% (N=15) | 0.079† |
| Duration corticosteroids (years) | 3.7 ± 2.5 | 2.6 ± 2.3 | 0.158* |
|
| |||
| Current or previous ACEi‡ | 39% (N=21) | 70% (N=16) | 0.014† |
| Duration ACEi (years) | 2.1 ± 1.8 | 4.1 ± 3.6 | 0.069* |
|
| |||
| Current or previous ARB§ | 6% (N=3) | 26% (N=6) | 0.010† |
| Duration ARB (years) | 3.8 ± 1.9 | 4.0 ± 2.0 | 0.794* |
|
| |||
| Current or previous ACEi or ARB | 41% (N=22) | 87% (N=20) | <0.001† |
| Duration ACEi + ARB (years) | 2.5 ± 2.0 | 4.5 ± 3.3 | 0.038* |
|
| |||
| Current or previous Beta blocker | 13% (N=7) | 70% (N=16) | <0.001† |
| Duration beta blocker (years) | 3.2 ± 2.6 | 3.4 ± 1.8 | 0.737* |
| Current or previous mineralocorticoid | 0 | 13% (N=3) | 0.006‖ |
| Duration of mineralocorticoid | 0 | 2 ± 1.5 | |
Mann-Whitney U test
Chi-Square test
Angiotensin converting enzyme inhibitor (ACEi)
Angiotensin receptor blocker (ARB)
Fisher’s exact test
Table 4.
Skeletal muscle strength at most recent measurement broken down by ambulatory status at time of measurement
| Subjective Strength | Non-ambulatory (N=21) |
Ambulatory (N=27) |
p-value |
|---|---|---|---|
| Current median subjective arm strength | 1 | 4 | <0.001* |
| Current median subjective leg strength | 0 | 4 | <0.001* |
|
| |||
| Mean number of QMT assessments | 7.4 ± 3.1 | 4.6 ± 2.8 | 0.004† |
| Current mean QMT (pounds) | 54.1 ± 28.1 | 102.6 ± 37.3 | <0.001† |
| Current mean arm QMT (pounds) | 20.0 ± 10.8 | 37.4 ± 15.5 | <0.001† |
| Current mean leg QMT (pounds) | 34.1 ± 18.4 | 65.3 ± 24.5 | <0.001† |
Chi square for a trend
Mann-Whitney U test
The generalized least square model allowed inclusion of repeated measures in assessing the relationship between skeletal muscle and cardiac function over time. Total QMT and arm QMT were correlated significantly with FS (both p=0.002). After adjustment for age, ambulatory status, and duration of corticosteroids and cardiac specific therapy, only total QMT remained significant (p=0.01) (Table 5). Further analysis demonstrated that the relationship between total QMT and FS is modified by ambulatory status (p for interaction term = 0.03, Figure 1). Among non-ambulatory boys, when total QMT increased from 55 pounds to 79 pounds, there was a 12.8% (95%CI: 5.2% to 21.0%, with p=0.001) relative increase in cardiac function (FS), while for ambulatory boys, the relationship between QMT and FS was not statistically significant (1.7% (95%CI: −5.4% to 9.2% with p value=0.65)).
Table 5.
Multivariable analysis of the effects of total quantitative muscle testing (QMT) on fractional shortening (FS) with adjustments for age, ambulatory status, duration of corticosteroids, and duration of cardiac-specific medications.
| Multivariable p-value |
|
|---|---|
| Total QMT | 0.01 |
| Ambulatory status | 0.08 |
| Duration of Cardiac-specific medications | 0.13 |
| Duration of Corticosteroids | 0.37 |
| Age | 0.19 |
Figure 1. Relationship between fractional shortening (FS) and quantitative muscle testing (QMT).
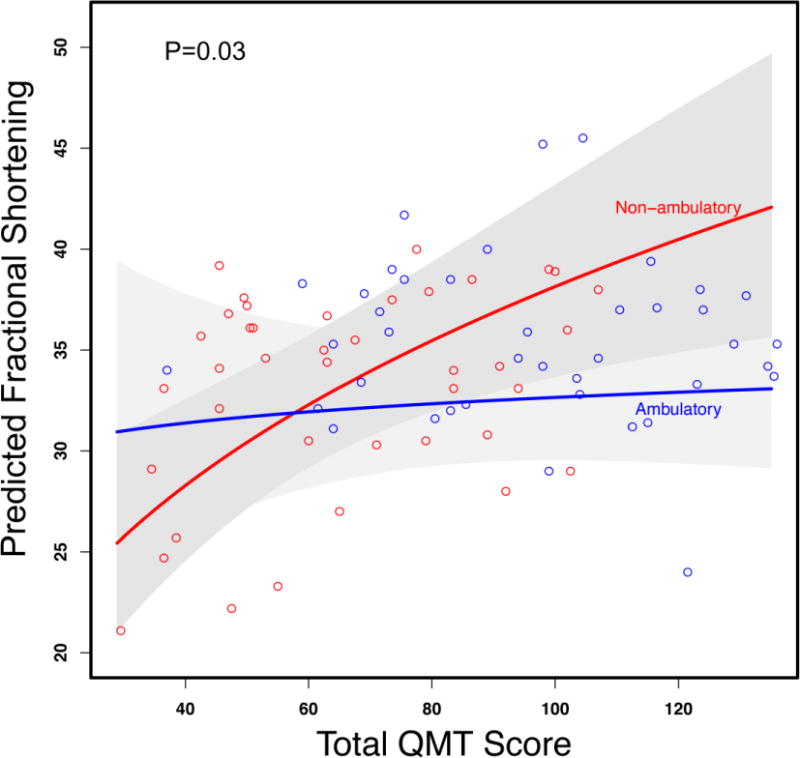
Figure 1 demonstrates predicted fractional shortening (FS) from the generalized least square model including the interaction between total quantitative muscle testing (QMT) and ambulatory status with adjustment for age and duration of corticosteroids and cardiac specific medications. To avoid over-fitting, only those risk factors deemed most important were pre-specified. This relationship is modified by ambulatory status (p=0.03 for interaction) such that greater QMT score was associated with a greater FS among non-ambulatory subjects while there is no significant relationship in ambulatory subjects.
4. DISCUSSION
This work is one of a few clinical evaluations investigating the longitudinal relationship between disease progression in both the skeletal muscle and cardiac function of boys with DMD. We report that the progression of skeletal muscle and cardiac disease in DMD is correlated in non-ambulatory boys. This is clinically significant because little is known about the effects of emerging DMD therapies on the myocardium. As novel therapies (e.g. exon skipping) become available, the indirect cardiac effects of improved skeletal muscle function will need to be monitored closely.
Our data suggest that preserved skeletal muscle function correlates with preserved cardiac function in non-ambulatory boys. These findings can impact the use of these medications and possibly inform future therapeutic trials. Our data also demonstrate a correlation between subjective muscle strength and cardiac function. We chose, however, to perform our primary analysis using QMT because it is an objective measure that is more reproducible. This is the first time, to our knowledge, that a direct correlation between preserved skeletal muscle function and cardiac muscle function has been demonstrated in non-ambulatory boys with DMD.
This relationship has been evaluated in animal models with mixed results. Townsend reported the development of acute dilated cardiomyopathy in mdx mice rescued with a skeletal-muscle specific transgene of mini-dystrophin.(9) Crisp demonstrated the opposite effect in both mdx mice and dystrophin/utrophin double knock out mice; their data suggested that selective rescue of diaphragmatic and skeletal muscle function with utrophin upregulation and exon-skipping led to restoration of RVEF and LVEF to wild type values.(19) Finally, Wasala examined skeletal muscle specific micro-dystrophin transgenic mice in old age and demonstrated no significant effect on myocardial fibrosis or cardiac function with skeletal muscle rescue.(20) Although experimental design differences such as murine strain, age, and method of rescue likely affected these study discrepancies, the magnitude of the difference between these study results highlights the challenge in understanding this key issue. Considering the conflicting animal data, human data will likely be necessary to ultimately answer this important question.
Given the important effects of corticosteroids and cardiac specific medications, controlling for these medications was integral to our current analysis. Corticosteroids improve skeletal muscle function and have been shown to delay the development of LV dysfunction and decrease mortality.(21–23) In addition, cardiac medications, such as angiotensin converting enzyme inhibitors, can delay onset of cardiac dysfunction when used prophylactically.(24) Future work should take into account the effects of both corticosteroids and cardiac specific medications.
Literature review identified only one study evaluating the relationship between skeletal muscle and cardiac myopathy progression in humans. Ergul demonstrated a direct correlation between the North Star Ambulatory Assessment (NSAA) and LVEF in a study limited to ambulatory DMD patients.(11) In contrast, we studied both ambulatory and non-ambulatory boys with DMD and demonstrated that ambulation modifies this relationship such that skeletal muscle strength is not correlated with cardiac function in DMD boys who remain ambulatory. One possible explanation for this discrepancy is that QMT and the NSAA are assessing different aspects of skeletal muscle function, the former assessing maximum voluntary isometric force and the latter using maneuvers centered on ambulation.
As this is a retrospective study, we are unable to conclude that there is a causal relationship between skeletal and cardiac muscle function. Therefore, there are multiple possible interpretations for these data. First there is a direct relationship between skeletal and cardiac muscle and that skeletal muscle modulates cardiac disease through endocrine signaling pathways. In this situation, we postulate that maintained ambulation increases myocardial demand, counteracting any potential positive signaling interactions from maintained skeletal muscle strength; once the myocardial demand of ambulation is removed, preserved skeletal muscle strength has a positive effect on cardiac function. Alternatively, there may be a direct relationship between skeletal and cardiac muscle, where improved cardiac function maintains skeletal muscle strength due to preserved cardiac output. While this explanation cannot be ruled out, it is unclear why this interaction is only present in non-ambulatory boys. Finally, the relationship between skeletal and cardiac muscle function may represents parallel progression of disease.
Although the precise relationship between skeletal and cardiac muscle function remains unclear, these data and those of Ergul et al. suggest that these processes are related. It must be emphasized that this correlation is moderate, both for subjective strength and QMT, and that there are patients with discordant skeletal muscle and cardiac function at both ends of the spectrum. Skeletal muscle strength cannot serve as a surrogate for cardiac function. As new therapeutics are evaluated, the cardiac effects must be monitored closely, even in clinical trials where skeletal muscle function is the primary endpoint.
Limitations
This is a retrospective cohort study in a relatively small number of subjects. As such, the results may not be completely generalizable. In addition, the significant correlation between skeletal muscle strength and cardiac function does not imply a causal relationship. Serial measures through a prospective evaluation would be needed to address this question. However, these findings do represent a novel relationship not yet described in DMD. We did not collect data prior to 1995 because of concerns over adequacy of medical record documentation as well as changes in treatment strategies and diagnostic equipment. It is possible that changes in treatment strategies and diagnostic equipment over that 18-year period will still lead to some bias in our results, but this long time period was utilized to improve power in this rare disease. We corrected for changes in treatment strategy by including duration of corticosteroids and cardiac-specific medications in our model.
Both QMT and FS have limitations as methods of assessing skeletal and cardiac function, but the ease of measurement and frequent use in other studies makes these measurements suitable for this study, as well as generalizable. Recent studies have demonstrated that FS is reproducible and correlates well with LVEF obtained by cardiac MRI.(12, 13) Future, prospective evaluation of this relationship should be performed using cardiac MRI as the modality for assessment of LV function.
Conclusions
In conclusion, we examined the relationship between skeletal and cardiac dysfunction in ambulatory and non-ambulatory DMD boys. In ambulatory subjects, there was no significant association between skeletal muscle strength and cardiac function. In contrast, in non-ambulatory subjects, higher skeletal muscle strength was significantly associated with preserved cardiac function. While the indirect cardiac effects of improved skeletal muscle function after therapies such as exon skipping have yet to be determined, emerging data suggest that skeletal and cardiac function are related. While further prospective evaluation is necessary, researchers must be vigilant in screening for cardiac effects of new therapies, even in clinical trials where skeletal muscle function is the primary endpoint.
Acknowledgments
The authors would like to acknowledge Stephen M. Damon for his help with data collection.
Funding:
This work was supported by American Heart Association Grant 13CRP14530007 (Soslow).
Research reported in this publication was supported by the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award Number K23HL123938 (Soslow). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The project was supported by the National Center for Research Resources, Grant UL1 RR024975-01, and is now at the National Center for Advancing Translational Sciences, Grant 2 UL1 TR000445-06. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
This project was supported by the Fighting Duchenne Foundation and the Fight DMD/Jonah & Emory Discovery Grant (Nashville, TN) (Markham).
None of the funding sources had input into the study design, collection, analysis and interpretation of data, the writing of the report, or the decision to submit the article for publication.
Abbreviations
- DMD
Duchenne muscular dystrophy
- LV
left ventricular
- LVEF
left ventricular ejection fraction
- FS
fractional shortening
- QMT
quantitative muscle testing
Footnotes
Conflict of Interest
The authors have no conflict of interest to report.
Contributor Information
Andrew D. Posner, Email: andrew.d.posner@gmail.com.
Jonathan H. Soslow, Email: jonathan.h.soslow@vanderbilt.edu.
W. Bryan Burnette, Email: william.b.burnette@vanderbilt.edu.
Aihua Bian, Email: anna.bian@vanderbilt.edu.
Ayumi Shintani, Email: ayumi.shintani@vanderbilt.edu.
Douglas B. Sawyer, Email: DSawyer@mmc.org.
Larry W. Markham, Email: larry.markham@vanderbilt.edu.
References
- 1.Dooley J, Gordon KE, Dodds L, MacSween J. Duchenne muscular dystrophy: a 30-year population-based incidence study. Clin Pediatr (Phila) 2010;49(2):177–9. doi: 10.1177/0009922809347777. [DOI] [PubMed] [Google Scholar]
- 2.Hoffman EP, Brown RH, Jr, Kunkel LM. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51(6):919–28. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- 3.Boland BJ, Silbert PL, Groover RV, Wollan PC, Silverstein MD. Skeletal, cardiac, and smooth muscle failure in Duchenne muscular dystrophy. Pediatr Neurol. 1996;14(1):7–12. doi: 10.1016/0887-8994(95)00251-0. [DOI] [PubMed] [Google Scholar]
- 4.Nigro G, Comi LI, Politano L, Bain RJ. The incidence and evolution of cardiomyopathy in Duchenne muscular dystrophy. International journal of cardiology. 1990;26(3):271–7. doi: 10.1016/0167-5273(90)90082-g. [DOI] [PubMed] [Google Scholar]
- 5.Eagle M, Baudouin SV, Chandler C, Giddings DR, Bullock R, Bushby K. Survival in Duchenne muscular dystrophy: improvements in life expectancy since 1967 and the impact of home nocturnal ventilation. Neuromuscul Disord. 2002;12(10):926–9. doi: 10.1016/s0960-8966(02)00140-2. [DOI] [PubMed] [Google Scholar]
- 6.Bach JR, Martinez D. Duchenne muscular dystrophy: continuous noninvasive ventilatory support prolongs survival. Respir Care. 2011;56(6):744–50. doi: 10.4187/respcare.00831. [DOI] [PubMed] [Google Scholar]
- 7.Petrof BJ. Molecular pathophysiology of myofiber injury in deficiencies of the dystrophin-glycoprotein complex. Am J Phys Med Rehabil. 2002;81(11 Suppl):S162–74. doi: 10.1097/00002060-200211001-00017. [DOI] [PubMed] [Google Scholar]
- 8.Spurney CF. Cardiomyopathy of Duchenne muscular dystrophy: current understanding and future directions. Muscle Nerve. 2011;44(1):8–19. doi: 10.1002/mus.22097. [DOI] [PubMed] [Google Scholar]
- 9.Townsend D, Yasuda S, Li S, Chamberlain JS, Metzger JM. Emergent dilated cardiomyopathy caused by targeted repair of dystrophic skeletal muscle. Molecular therapy. 2008;16(5):832–5. doi: 10.1038/mt.2008.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saito M, Kawai H, Akaike M, Adachi K, Nishida Y, Saito S. Cardiac dysfunction with Becker muscular dystrophy. Am Heart J. 1996;132(3):642–7. doi: 10.1016/s0002-8703(96)90250-1. [DOI] [PubMed] [Google Scholar]
- 11.Ergul Y, Ekici B, Nisli K, Tatli B, Binboga F, Acar G, et al. Evaluation of the North Star Ambulatory Assessment scale and cardiac abnormalities in ambulant boys with Duchenne muscular dystrophy. J Paediatr Child Health. 2012;48(7):610–6. doi: 10.1111/j.1440-1754.2012.02428.x. [DOI] [PubMed] [Google Scholar]
- 12.Spurney CF, McCaffrey FM, Cnaan A, Morgenroth LP, Ghelani SJ, Gordish-Dressman H, et al. Feasibility and Reproducibility of Echocardiographic Measures in Children with Muscular Dystrophies. J Am Soc Echocardiogr. 2015;28(8):999–1008. doi: 10.1016/j.echo.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brunklaus A, Parish E, Muntoni F, Scuplak S, Tucker SK, Fenton M, et al. The value of cardiac MRI versus echocardiography in the pre-operative assessment of patients with Duchenne muscular dystrophy. Eur J Paediatr Neurol. 2015;19(4):395–401. doi: 10.1016/j.ejpn.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 14.Mathur S, Lott DJ, Senesac C, Germain SA, Vohra RS, Sweeney HL, et al. Age-related differences in lower-limb muscle cross-sectional area and torque production in boys with Duchenne muscular dystrophy. Arch Phys Med Rehabil. 2010;91(7):1051–8. doi: 10.1016/j.apmr.2010.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lerario A, Bonfiglio S, Sormani M, Tettamanti A, Marktel S, Napolitano S, et al. Quantitative muscle strength assessment in duchenne muscular dystrophy: longitudinal study and correlation with functional measures. BMC Neurol. 2012;12:91. doi: 10.1186/1471-2377-12-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bushby K, Finkel R, Birnkrant DJ, Case LE, Clemens PR, Cripe L, et al. Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and pharmacological and psychosocial management. The Lancet Neurology. 2010;9(1):77–93. doi: 10.1016/S1474-4422(09)70271-6. [DOI] [PubMed] [Google Scholar]
- 17.Bushby K, Finkel R, Birnkrant DJ, Case LE, Clemens PR, Cripe L, et al. Diagnosis and management of Duchenne muscular dystrophy, part 2: implementation of multidisciplinary care. The Lancet Neurology. 2010;9(2):177–89. doi: 10.1016/S1474-4422(09)70272-8. [DOI] [PubMed] [Google Scholar]
- 18.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crisp A, Yin H, Goyenvalle A, Betts C, Moulton HM, Seow Y, et al. Diaphragm rescue alone prevents heart dysfunction in dystrophic mice. Hum Mol Genet. 2011;20(3):413–21. doi: 10.1093/hmg/ddq477. [DOI] [PubMed] [Google Scholar]
- 20.Wasala NB, Bostick B, Yue Y, Duan D. Exclusive skeletal muscle correction does not modulate dystrophic heart disease in the aged mdx model of Duchenne cardiomyopathy. Hum Mol Genet. 2013;22(13):2634–41. doi: 10.1093/hmg/ddt112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Griggs RC, Moxley RT, 3rd, Mendell JR, Fenichel GM, Brooke MH, Pestronk A, et al. Prednisone in Duchenne dystrophy. A randomized, controlled trial defining the time course and dose response. Clinical Investigation of Duchenne Dystrophy Group. Arch Neurol. 1991;48(4):383–8. doi: 10.1001/archneur.1991.00530160047012. [DOI] [PubMed] [Google Scholar]
- 22.Markham LW, Kinnett K, Wong BL, Woodrow Benson D, Cripe LH. Corticosteroid treatment retards development of ventricular dysfunction in Duchenne muscular dystrophy. Neuromuscul Disord. 2008;18(5):365–70. doi: 10.1016/j.nmd.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 23.Schram G, Fournier A, Leduc H, Dahdah N, Therien J, Vanasse M, et al. All-cause mortality and cardiovascular outcomes with prophylactic steroid therapy in Duchenne muscular dystrophy. J Am Coll Cardiol. 2013;61(9):948–54. doi: 10.1016/j.jacc.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 24.Duboc D, Meune C, Pierre B, Wahbi K, Eymard B, Toutain A, et al. Perindopril preventive treatment on mortality in Duchenne muscular dystrophy: 10 years’ follow-up. Am Heart J. 2007;154(3):596–602. doi: 10.1016/j.ahj.2007.05.014. [DOI] [PubMed] [Google Scholar]


