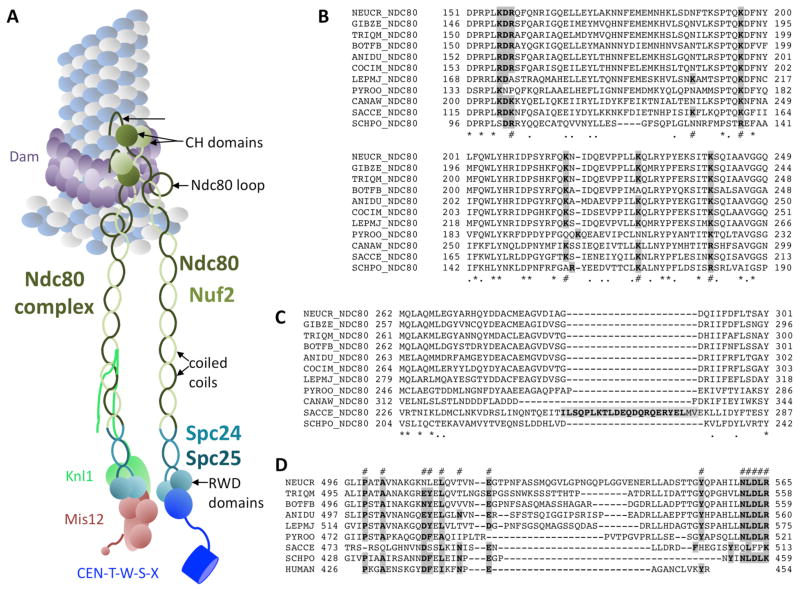
FIG. 3.
The Ndc80 complex connects microtubules (MT) to the Mis12 assembly and the CCAN CENP-T complex. A. Structural features of the Ndc80 complex. N-terminal, positively charged patches bind directly to the MT. Calponin-homology (CH) domains are well-conserved and essential for MT binding. The function of the exposed Ndc80 loop is still unclear. All four Ndc80 subunits (Ndc80, Nuf2, Spc24, Spc25) contain regions of coiled-coils that connect the two head units, the CH domains of Ndc80/Nuf2 and the heterodimeric RWD domain of Spc24/Spc25. Binding to the RWD domain by Mis12 (and Knl1) or CENP-T is exclusive – only one of these complexes can bind to one Spc24/Spc25 RWD domain. B. Alignment of a conserved regions within the Ndc80 CH domain, containing lysine (K) residues previously shown to be important for function (#). Completely conserved residues (*) or similar (.) residues are marked. Double mutants for the first (K122E) and last (K204E) lysine in yeast were unable to survive, and mutants with only K204E showed benomyl sensitivity. C. A short loop separating coiled-coil regions of Ndc80 in some yeast species is not found in filamentous ascomycetes. Instead these regions are highly conserved. D. The exposed Ndc80 loop varies in length and primary sequence but several residues are highly conserved (#). Mutational analyses of the features shown in B. – D. needs to be carried out for filamentous ascomycetes. For species abbreviations see SUPPLEMENTAL TABLE 1. Important residues are shown in bold and shaded in grey.