Abstract
Background
Cachexia has a devastating impact on survival and quality of life for many cancer patients and contributes to nearly one‐third of all cancer deaths; also, it is associated with poor responses to chemotherapy and survival. A better understanding of the underlying mechanisms of cancer‐associated cachexia (CAC), coupled with effective therapeutic approaches, will improve management of progressive functional impairment in cancer patients. Salidroside, a phenylpropanoid glycoside in Rhodiola rosea L, has been reported to possess potential anti‐fatigue, anti‐ageing, and anti‐Alzheimer's disease properties. It is widely consumed as a nutritional supplement, but its effects on CAC and the possible mechanism remain a mystery.
Methods
In the murine models of cachexia induced by CT‐26 and Lewis lung carcinoma (LLC) tumour, respectively, main features of CAC were determined after treatment of salidroside or chemotherapy. In vitro experiments were performed using murine C2C12 myotubes, which were treated by tumour necrosis factor‐α. Levels of several critical muscle‐related signal proteins such as mammalian target of rapamycin (mTOR), p‐mTOR, and myosin heavy chain (MyHC) were examined using western blot both in vitro and in vivo.
Results
In the present study, we showed the exciting effect of salidroside on the treatment of CAC. In CT‐26 and LLC models, respectively, salidroside treatment could effectively preserve the tumour‐free body weight, decrease loss of adipose and gastrocnemius muscles, alleviate tumour burden, and prolong their survival time. Additionally, in combined chemotherapy, salidroside could synergistically enhance the anti‐tumour activity of cisplatin, especially decreased or eliminated chemotherapy‐induced cachexia. Further analysis demonstrated that salidroside could significantly increase expression of mTOR, p‐mTOR, and MyHC in gastrocnemius muscle. Also, results in vitro showed that salidroside could not only obviously increase mTOR, p‐mTOR, and MyHC expression in C2C12 myotubes but also effectively rescue their down‐regulation induced by tumour necrosis factor‐α.
Conclusions
In the current study, the exciting effect of salidroside on CAC suggested that salidroside supplementation might be a promising approach for a multi‐targeted therapy for the treatment of CAC.
Keywords: Salidroside, Cancer‐associated cachexia, Skeletal muscle, mTOR, MyHC
Introduction
Cachexia is a multi‐factorial wasting syndrome that not only has a dramatic impact on patient quality of life through skeletal muscle wasting but also is associated with poor responses to chemotherapy and survival.1, 2, 3 In 2014, current statistics showed that a total of 1 665 540 new cancer cases and 585 720 deaths from cancer are projected to occur in the USA.4 However, up to 80% of cancer patients suffer from cachexia, resulting in weight loss, a reduced quality of life, and a shortened survival time. And cachexia contributes to nearly one‐third of all cancer deaths.1, 2, 5 Most commonly, it is characterized by a significant reduction in body weight resulting predominantly from loss of adipose tissue and skeletal muscle, and in a formal consensus process, cancer‐associated cachexia (CAC) was defined as a multi‐factorial syndrome defined by an ongoing loss of skeletal muscle mass (with or without loss of fat mass).6, 7, 8, 9, 10 Although appetite stimulants or nutritional support could help reverse the loss of fat, the reversal of muscle mass wasting is more difficult and remains a challenge in CAC patient care.11, 12 A better understanding of the underlying mechanisms, coupled with effective treatment options, will improve management of wasting in cancer patients.13 Recently, growing evidence has shown that natural products, especially plant products, display chemopreventive effects and play key roles in prevention and treatment of disease.14 And attention has been directed towards natural products found in certain herbs as treatment of CAC.15, 16, 17 Salidroside, one of the major phenylpropanoid glycosides found in Rhodiola rosea L, is consumed almost daily in many fields worldwide, especially in cosmetics and food additives. Previous studies showed that salidroside has been identified possessing potential anti‐fatigue and anoxia,18 anti‐ageing,19 and anti‐Alzheimer's disease properties.20, 21 Here, we showed the exciting effect of salidroside on CAC and its possible mechanism both in vitro and in vivo.
Results
Anti‐cachexia efficacy of salidroside in CT‐26 or LLC model
In 2011, a panel of experts participated in a formal consensus process to develop a framework for the definition and classification of CAC. Finally, in the international consensus, CAC was defined as a multi‐factorial syndrome characterized by an ongoing loss of body weight and skeletal muscle mass (with or without loss of fat mass).6 Here, to assess the anti‐cachexia potential of salidroside, we used two different cachexia models in mice induced by CT‐26 and LLC tumour, respectively. As shown in Figures 1 and 2, two models of CAC were established, respectively, which were characterized by obvious decreases of total body weight, tumour‐free body weight and food intake, and significant loss of gastrocnemius muscle and fat mass compared with the negative group. Excitingly, salidroside could distinctly improve chief features of CAC in a dose‐dependent manner. First, in CT‐26 CAC model, compared with the control group, although the salidroside‐treated groups just showed a certain degree of anti‐tumour effect, the mean tumour volume was diminished by approximately 50% even at dose of 120 mg/kg (Figure 1B), and the difference in total body weight was also not significant (Figure 1C); salidroside could significantly preserve fat mass (Figure 1A) and increase the tumour‐free body weight (Figure 1D), especially the mass of gastrocnemius muscles (Figure 1E) in a dose‐dependent manner, which implied that salidroside could distinctly improve main features of CAC in mice. Additionally, compared with the control group, salidroside‐treated groups showed obvious improvement of food intakes, as shown in Figure 1F; on 16th day, clear differences were found between salidroside‐treated groups and the control group, and finally, food intakes of salidroside‐treated groups tend to be much more than the control group. Furthermore, general lifetime measurements were performed, and results showed that salidroside could significantly extend their survival time (Figure 1G). These results showed that salidroside had an effective anti‐cachexia activity in CT‐26 CAC model. Second, in LLC CAC model, consistent with the data above, the similar results were obtained (Figure 2A–G). Taken together, these results indicated that salidroside had an effective anti‐cachexia activity in CAC models in vivo, including increasing quality of life and prolonging survival time.
Figure 1.
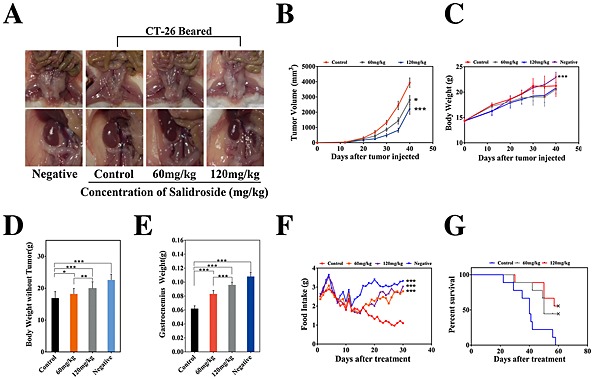
Therapeutic efficacy of salidroside in the model of cachexia induced by CT‐26. Cachexia was induced in BABL/c mice by subcutaneous injection of CT‐26 cells, which were characterized by obvious decreases of body weight and food intake and significant loss of gastrocnemius muscle and fat mass compared with the negative control. Tumour‐bearing mice were treated with NS, salidroside (60 mg/kg/day), and salidroside (120 mg/kg/day), respectively. Effects of salidroside on the main features of cachexia were examined, including (B) tumour growth, (C) body weight, (D) tumour‐free body weight, (E) gastrocnemius muscle, (A) fat mass (bottom, epididymal WAT; top panel, gonadal WAT), (F) food intake, and (G) survival. Data were presented as mean ± SEM (*P < 0.05, **P < 0.01, ***P < 0.001). WAT, white adipose tissue. Negative, the healthy BABL/c mice.
Figure 2.
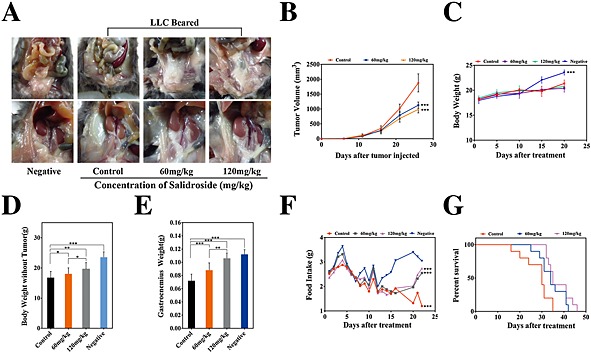
Therapeutic efficacy of salidroside in the model of cachexia induced by LLC. Like CT‐26 model above, effects of salidroside on the main features of cachexia were examined, including (B) tumour growth, (C) body weight, (D) tumour‐free body weight, (E) gastrocnemius muscle, (A) fat mass (bottom, epididymal WAT; top, gonadal WAT), (F) food intake, and (G) survival. Data were presented as mean ± SEM (*P < 0.05, **P < 0.01, ***P < 0.001). WAT, white adipose tissue. Negative, the healthy C57BL/6 mice.
Anti‐cachexia efficacy of salidroside in combined chemotherapy
To further investigate the anti‐cachexia effect of salidroside in vivo, combined chemotherapy was performed in the models of cachexia induced by CT‐26 and LLC tumour, respectively. As shown in Figure 3, in CT‐26 CAC model, salidroside could not only still exert significant and stable anti‐cachexia effects like above but also assist to enhance anti‐tumour activity of cisplatin (DDP). Here, although not statistically significant, to a certain degree, we observed that tumour‐free body weight and gastrocnemius muscle weight tended to be lower in DDP‐treated groups than the control group (Figure 3D, E), implying that DDP might induce some degree of cachexia to mice at the concentrations employed in this study. Notably, different from the anti‐cachexia activity of salidroside, when DDP significantly inhibited the growth of tumour (Figure 3B), it resulted in obviously loss of total body weight (Figure 3C) and fat mass (Figure 3A) as compared with the control group, both of them were consistent with main features of CAC. Excitingly, compared with the DDP group or salidroside group, the combined therapy group showed much better effects on cachexia, suggesting that salidroside could not only significantly enhance anti‐tumour activity of DDP (Figure 3B) but also obviously alleviate DDP‐induced cachexia, including preservation of total body weight (Figure 3C), tumour‐free body weight (Figure 3C, D), adipose and gastrocnemius muscle mass (Figure 3A, E), and assist DDP to prolong survival time of mice (Figure 3F). Additionally, pathologic analysis showed that salidroside could significantly ameliorate or eliminate the DDP‐induced nephrotoxicity, suggesting that it had a good capability of reducing side effects in tumour chemotherapy in vivo (Supporting Information Figure 1). Furthermore, in LLC CAC model, consistent with data in CT26 CAC model, the similar results were also obtained (Figure 4A–F, Supporting Information Figure 1). Collectively, the results above showed that the combined therapy was proved more effective, which prolonged survival and improved quality of life in CAC models compared with other groups, demonstrating the ability of salidroside to assist DDP to exert more effective anti‐cachexia activity in vivo.
Figure 3.
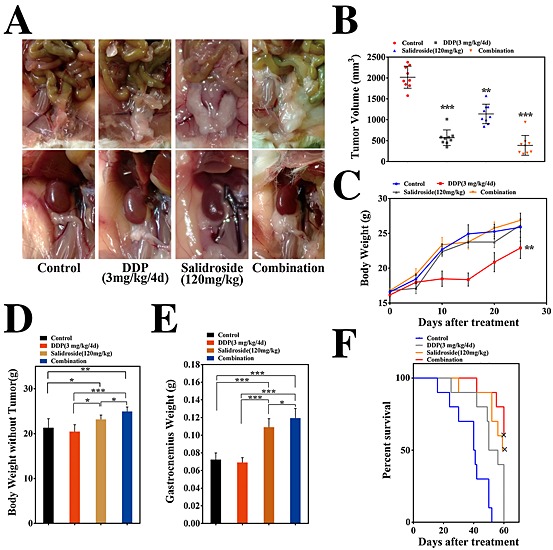
Anti‐cachexia efficacy of salidroside in the combined chemotherapy in CT‐26 models. Here, cachexia model induced by CT‐26 was established again, and further study of combined chemotherapy was performed to focus evaluations of anti‐cachexia efficacy of salidroside. Tumour‐bearing mice were treated with NS, DDP (3 mg/kg/4 days), salidroside (120 mg/kg/day), and combination of salidroside (120 mg/kg/day) and single dose of DDP (3 mg/kg/4 days), respectively. Effects of salidroside on the main features of cachexia were examined, including (B) tumour growth, (C) body weight, (D) tumour‐free body weight, (E) gastrocnemius muscle, (A) fat mass (bottom, epididymal WAT; top panel, gonadal WAT), and (F) survival. Results are expressed as mean ± SEM (*P < 0.05, **P < 0.01, ***P < 0.001). WAT, white adipose tissue; NS, the control group; DDP, cisplatin.
Figure 4.
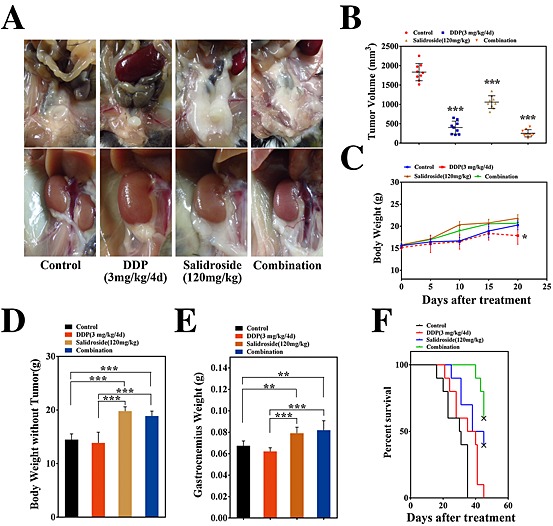
Anti‐cachexia efficacy of salidroside in the combined chemotherapy in LLC models. Like CT‐26 model in combined chemotherapy above, here, LLC models were established again, and the combined chemotherapeutic efficacy of salidroside and DDP was examined with focusing evaluations of anti‐cachexia efficacy of salidroside. Groups were designed like the combined chemotherapy in CT‐26 models. Anti‐cachexia efficacy of salidroside was determined, including (B) tumour growth, (C) body weight, (D) tumour‐free body weight,(E) gastrocnemius muscle, (A) fat mass (bottom, epididymal WAT; top, gonadal WAT), and (F) survival. Results are expressed as mean ± SEM (*P < 0.05, **P < 0.01, ***P < 0.001). WAT, white adipose tissue; NS, the control group; DDP, cisplatin.
Up‐regulation of mTOR, p‐mTOR, and MyHC expression induced by salidroside in gastrocnemius muscle
All these data above suggested that salidroside could effectively improve CAC in vivo. To further investigate the possible anti‐cachexia mechanism of salidroside, the levels of several critical muscle‐related signal proteins such as mammalian target of rapamycin (mTOR), p‐mTOR, and myosin heavy chain (MyHC) in gastrocnemius muscle were determined by western blot. Previous studies have indentified that loss of mTOR in skeletal muscle could result in a severe myopathy, thereby further extending the list of disorders related to mTOR dysfunction.22, 23 Here, as shown in Figure 5C, D, our result showed that salidroside could significantly increase expressions of mTOR, p‐mTOR, and MyHC in gastrocnemius muscle compared with the control group, suggesting that it is possible that salidroside could improve CAC via increasing mTOR, p‐mTOR, and MyHC expression and thereby improving muscle nutrition.
Figure 5.
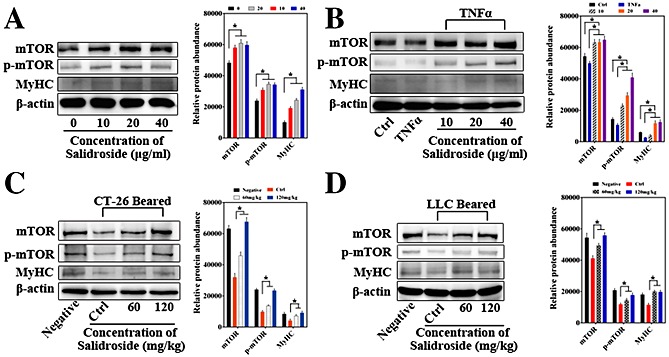
The possible anti‐cachexia mechanism of salidroside. Levels of several critical muscle‐related signal proteins including mTOR, p‐mTOR, and MyHC in gastrocnemius muscle and C2C12 myotubes were determined by western blot. (A) Effect of salidroside on mTOR, p‐mTOR, and MyHC expression in C2C12 myotubes, which were treated by salidroside at different concentrations (10, 20, and 40 µg/mL) without serum for 24 h. (B) C2C12 myotubes were stimulated with TNF‐α (50 ng/mL) for 6 h and then treated by salidroside at different concentrations (10, 20, and 40 µg/mL) for 24 h, and then mTOR, p‐mTOR, and MyHC expressions were determined by western blot. (C, D) Up‐regulation of mTOR, p‐mTOR, and MyHC expression induced by salidroside in gastrocnemius muscle in the model of cachexia induced by CT‐26 and LLC as compared with the control group, respectively. Pairs of total protein were normalized by β‐actin. Correspondingly, quantification of western blot analysis images was performed using Image J software (NIH, Bethesda, MD, USA) from three or more independent experiments and was presented in graphical form (*P < 0.05). Statistics were carried out with a two‐tailed Student's t‐test. Ctrl, the control group; DDP, cisplatin; mTOR, mammalian target of rapamycin; MyHC, myosin heavy chain; TNF‐α, tumour necrosis factor‐α.
Effect of salidroside on mTOR, p‐mTOR, and MyHC expression in C2C12 myotubes
To further determine the effect of salidroside in vitro, firstly, proliferation of C2C12 myoblast cells treated by salidroside was examined using CCK‐8 assay. Although not statistically significant, to a certain degree, we observed salidroside promoted proliferation of C2C12 myoblast cells, and the OD values determined by the CCK‐8 tended to be higher in salidroside‐treated groups than the control group (data not shown), implying that there was no evidence for toxicity of salidroside to myoblast cells at any of the concentrations employed in this study. Additionally, the C2C12 murine myoblast cells were induced by 2% horse serum to differentiate into myotubes, and as illustrated in Figure 5A, results showed that salidroside could significantly raise levels of mTOR, p‐mTOR, and MyHC in murine myotubes.
Effect of salidroside on mTOR, p‐mTOR, and MyHC expression in C2C12 myotubes in the presence of TNF‐α
Additionally, to further verify the effects above, in a model of muscle atrophy in vitro that has been proved valid by Helen L. Eley24 and Kamran A. Mirza,15 as illustrated in Figure 5B, our results showed that levels of mTOR, p‐mTOR, and MyHC were significantly decreased in myotubes treated with tumour necrosis factor‐α (TNF‐α) (50 ng/mL) for 6 h, and of note, all of which were also effectively rescued by salidroside treatment for 24 h, suggesting that it is possible that salidroside could improve muscle nutrition via increasing mTOR, p‐mTOR, and MyHC expression.
Discussion
Cachexia (Greek for ‘bad condition’) is a devastating syndrome most common in patients with cancer, which has a dramatic impact on patient quality of life and survival.25, 26 The most important features of cachexia are a significant reduction in body weight and loss of skeletal muscle mass (with or without loss of fat mass),2, 3, 9, 11 implying a tumour‐associated metabolic condition that specifically targets muscle.25, 27, 28 Because weight loss shortens the survival time of CAC patients and reduces performance status, effective therapy would prolong patients survival and improve quality of life.12 In recent years, attention has been directed towards natural products found in certain herbs as treatment of CAC. Salidroside, one of the major phenylpropanoid glycosides found in R. rosea L, is consumed almost daily as a nutritional supplement in many countries and has been identified possessing potential anti‐fatigue and anoxia,18 anti‐aging,19 and anti‐Alzheimer's disease activities.20, 21 To investigate the anti‐cachexia effect of salidroside and its possible mechanism, in the present study, experiments both in vitro and in vivo were performed, and results have showed that salidroside treatment could effectively improve major characteristics of CAC. In general, in two CAC models, results indicated that salidroside preserved the tumour‐free body weight, improved the mass of gastrocnemius muscles and food intake, decreased loss of adipose, and importantly, extended their survival time. However, salidroside just showed some degree of anti‐tumour effect even at dose of 120 mg/kg, suggesting that salidroside‐exerted anti‐cachexia effect did not seem to be solely decreasing tumour size but might be nutritional supplementation on muscle. Besides, some of the findings have been noteworthy; in combined therapy, salidroside displayed exciting effects, and it could synergistically enhance the anti‐tumour activity of DDP, especially decrease or eliminate DDP‐induced cachexia, like improving tumour‐free body weight and loss of adipose and gastrocnemius muscle mass and prolonging survival time. Notably, although DDP is used extensively for the treatment of a variety of solid tumours,29 clinical data showed many substantial short‐term and long‐term side effects, which affected the quality of life in cancer patients, and these chemotherapy‐induced toxicities need to be recognized, prevented, and optimally managed. In recent years, many researchers or institutions appeal for paying more attention to quality of life in cancer patients than just killing cancer itself.30 Here, our data also indicated that DDP‐induced cachexia, including loss of body weight, tumour‐free body weight, fat and gastrocnemius muscle mass, and the combined therapy, was proved more effective, which prolonged survival and improved quality of life compared with other groups in CAC models; additionally, pathologic analysis showed that salidroside could significantly ameliorate or eliminate the DDP‐induced nephrotoxicity. All of which displayed the ability of salidroside to assist DDP to exert more effective anti‐cachexia activity in vivo, suggesting that salidroside might be clinically efficacious as promising additions to therapeutic strategies for CAC patients.
Given that muscle structure, mass, and composition are critical for motility, whole body metabolism, and viability, and in CAC, muscle atrophy commonly occurs, and its loss could result in weakness and inability to carry out normal activities, and even death due to multiple organ failure.23 Definition of CAC also underscores that loss of skeletal muscle mass is the most important element of its major characteristics.6 Hence, drugs targeting skeletal muscle‐related pathway hold promise in treating CAC. As a conserved Ser/Thr kinase, mTOR is a central regulator of cell growth by integrating signals from nutrients, growth factors, and energy status, and Valérie Risson et al. have demonstrated that the loss of mTOR in skeletal muscle could result in a severe myopathy, thereby further extending the list of disorders related to mTOR dysfunction.22 To deeply explore the anti‐cachexia mechanism of salidroside, the levels of several critical muscle‐related signal proteins such as mTOR, p‐mTOR, and MyHC were determined in gastrocnemius muscle in two CAC models. On the one hand, in the models of cachexia induced by CT‐26 and LLC tumour, respectively, our findings indicated that levels of mTOR, p‐mTOR, and MyHC were significantly decreased in gastrocnemius muscle while effectively rescued by salidroside treatment, suggesting that down‐regulation of mTOR and p‐mTOR expression might contribute to these disorders, consistent with the previous studies that have indentified that mTOR inhibition could cause decreases in body weight, food intake, and fat mass in the rodent models.31 On the other hand, although not statistically significant, to a certain degree, we observed that salidroside promoted proliferation of C2C12 myoblast cells in vitro (data not shown), implying that there was no evidence for toxicity of salidroside to myoblast cells at any of the concentrations employed in this study. Correspondingly, we found that salidroside could significantly raise mTOR, p‐mTOR, and MyHC levels in C2C12 murine myotubes. Additionally, in the further experiments, in a model of muscle atrophy in vitro that has been proved valid by Helen L. Eley18 and Kamran A. Mirza,9 our results also showed that salidroside could effectively rescue the significantly decreased levels of mTOR, p‐mTOR, and MyHC in myotubes induced by TNF‐α (50 ng/mL), implying that it is possible that salidroside could improve muscle nutrition via increasing mTOR, p‐mTOR, and MyHC expression.
Collectively, our study revealed the exciting effect of salidroside for the treatment of CAC, and the improvement of CAC by salidroside might contribute to its ability to up‐regulate mTOR, p‐mTOR, and MyHC expression; it is possible that salidroside proves clinically efficacious as valuable additions to multi‐targeted therapeutic strategies for CAC patients.
Materials and methods
Reagents and antibodies
The primary antibodies against mTOR, p‐mTOR, and β‐actin were acquired from Cell Signaling Technology (Beverly, MA) and antibodies against MyHC from Abcam (Cambridge, USA). Anti‐mouse and anti‐rabbit IgG antibodies conjugated with horseradish peroxidase were purchased from Zhongshan Biological (Beijing, China). Salidroside (purity > 98%) provided by Chengdu Must Bio‐technology Co. Ltd (China) was prepared in medium (serum free) at a concentration of 3 mg/mL until use.
Cell lines and culture
The murine colon tumour cell line CT‐26 and murine Lewis lung carcinoma cell line LLC were obtained from the American Type Culture Collection (ATCC, Manassas, VA), and C2C12 myoblast cell line was from The Cell Bank of Type Culture Collection of Chinese Academy of Sciences. Cells were cultured in either Dulbecco's modified Eagle's minimal essential medium or RPMI‐1640 (Gibco, USA) supplemented with 10% foetal bovine serum (Biowest, France), 100 units/mL penicillin, and 100 units/mL streptomycin at 37 °C, in a humidified atmosphere containing 5% CO2. To generate C2C12 myotubes, confluent cultures of C2C12 cells were grown in Dulbecco's modified Eagle's minimal essential medium with 2% horse serum for 6–8 days, with medium changes every 2 days, and the myotubes remained viable for a further 2–3 days. We found that C2C12 myotubes generated from early passage (<10 passages after purchase) C2C12 myoblast cells yielded most consistent results.
Western blotting
Muscle tissues from animals were ground in liquid nitrogen, and then proteins were extracted using M‐PER ®Mammalian Protein Extraction Reagent (Pierce, Thermo), following the manufacturer's instructions and quantified by the Nanno Drop 2000/2000c (Thermo). Samples were separated by 8% or 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis, transferred to polyvinylidene fluoride membranes (Millipore), and the membrane was blocked in 5% BSA at 37 °C for 2 h and subsequently probed by the following primary antibodies: anti‐MyHC (diluted 1:1000; Abcam) and anti‐mTOR/p‐mTOR (diluted 1:2000; Cell Signalling Technology). Blots were incubated with the respective primary antibodies overnight at 4 °C. After washing three times in Tris‐buffered saline with Tween 20, the blots were incubated with secondary antibody (diluted 1:10000; Zhongshan Biological) conjugated to horseradish peroxidase for 2 h at 37 °C. Finally, blots were visualized by enhanced chemiluminescence reagents (Amersham Biosciences). β‐Actin was used as an internal control.
Animal studies
All animal care and experimental procedures were approved by the Institutional Animal Care and Treatment Committee of Sichuan University. Four to five weeks old healthy female BABL/c and C57BL/6 mice were purchased from the Institute of Laboratory Animal Sciences, CAMS&PUMC (Beijing, China). The animals were maintained in conditions of constant temperature and humidity with a regular light : dark cycle (light on from 08:00 a.m. to 08:00 p.m.). BABL/c mice were inoculated with CT‐26 cells (1.0 × 106/100 μL), while C57BL/6 mice with LLC cells (7 × 105/100 μL), both of them were injected s.c. into the right flank. Nine days after inoculation, tumours were palpable (about 5 mm in diameter) in BABL/c and C57BL/6 mice, respectively. Tumour‐bearing mice were randomly divided into three groups (n = 9 per group). In a separate survival experiment with BABL/c and C57BL/6 mice (n = 10 mice per group), survival time of mice was observed to evaluate the life‐prolonging effect. Treatments were given i.p. with the control group, salidroside (60 mg/kg/day), and salidroside (120 mg/kg/day) for 25 days in BABL/c tumour model, while for 20 days in C57BL/6 model. Tumour volumes were calculated using the formula: tumour volume (mm3) = 0.52 × length × width2 in which length and perpendicular width were measured by a vernier calliper. The body weights of the mice were measured every 5‐day post‐tumour implantation, and food intake was measured daily. In the end, mice were sacrificed, both tumours and gastrocnemius muscles were removed, weighed, and quickly frozen in liquid nitrogen for subsequent analysis. Tissues of kidney from mice of each group were fixed in 4% polyformaldehyde, paraffin embedded, then 4‐µm‐thick sections were stained with haematoxylin/eosin, and histopathological changes were evaluated. Also, gonadal and epididymal adipose tissue was also determined by visual inspection.
Combined chemotherapy
CT‐26 and LLC models were established again to have further studies of the combined chemotherapeutic efficacy of salidroside and DDP. For this reason, we still focused the evaluations of anti‐cachexia efficacy of salidroside. Tumours were generated according to the method previously described by CT‐26 and LLC tumour models. The different thing was that four experimental arms were studied: (i) animals receiving control group only (control); (ii) animals receiving salidroside (120 mg/kg/day) alone; (iii) animals receiving a single DDP alone (3 mg/kg/4 days); and (iv) animals receiving a combination of salidroside (120 mg/kg/day) and single dose of DDP (3 mg/kg/4 days). The groups consisted of nine mice each; different from salidroside, DDP was given i.p. for 25 or 20 days (3 mg/kg/4 days), respectively. Finally, the same measurements above were performed. Also, in a separate survival experiment with BABL/c and C57BL/6 mice, survival time of mice was observed to evaluate the life‐prolonging effect.
Statistical analysis
All values were reported as means + SEM. Statistical analysis was performed using GraphPad Prism 6 software. Normal distribution and homogeneity of variances were performed using Shapiro–Wilk test and Levene's test in SPSS Statistics, respectively. Comparisons between values from two groups were performed using Student's t‐tests and multiple groups by analysis of variance. Significance level was set at P < 0.05.
Acknowledgements
The authors thank Juan He for helpful suggestions in in vivo studies and Xuewen Jin for excellent discussions in in vitro studies and data analysis. The authors of this manuscript certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle 2010, 1:7–8 (von Haeling S, Morley J. E., Coats A. J., and Anker S. D.).
Conflicts of interest
The authors declare that no potential conflicts of interest were disclosed.
Supporting information
Supplement 1. Histopathological changes of kidney of each group in the combined chemotherapies in CT‐26 (Left) and LLC models (Right), respectively. Tissues of kidney from mice of each group were fixed in 4% polyformaldehyde, paraffin‐embedded, and then 4‐µm‐thick sections were stained with hematoxylin/eosin (HE). The right panel of each CAC model depicted higher magnifications of them
Supporting info item
Chen, X. , Wu, Y. , Yang, T. , Wei, M. , Wang, Y. , Deng, X. , Shen, C. , Li, W. , Zhang, H. , Xu, W. , Gou, L. , Zeng, Y. , Zhang, Y. , Wang, Z. , and Yang, J. (2016) Salidroside alleviates cachexia symptoms in mouse models of cancer cachexia via activating mTOR signalling. Journal of Cachexia, Sarcopenia and Muscle, 7: 225–232. doi: 10.1002/jcsm.12054.
References
- 1. Dewys WD, Begg C, Lavin PT, Band PR, Bennett JM, Bertino JR, et al. Prognostic effect of weight loss prior to chemotherapy in cancer patients. Am J Med 1980; 69: 491–497. [DOI] [PubMed] [Google Scholar]
- 2. Deans C, Wigmore SJ. Systemic inflammation, cachexia and prognosis in patients with cancer. Curr Opin Clin Nutr Metab Care 2005; 8: 265–269. [DOI] [PubMed] [Google Scholar]
- 3. Tan BH, Fearon KC. Cachexia: prevalence and impact in medicine. Curr Opin Clin Nutr Metab Care 2008; 11: 400–407. [DOI] [PubMed] [Google Scholar]
- 4. Siegel R, Ma JM, Zou ZH, Jemal A. Cancer statistics. CA Cancer J Clin 2014; 64: 9–29. [DOI] [PubMed] [Google Scholar]
- 5. Fearon KC, Moses AG Cancer cachexia. Int J Cardiol 2002; 85: 73–81. [DOI] [PubMed] [Google Scholar]
- 6. Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 2011; 12: 489–495. [DOI] [PubMed] [Google Scholar]
- 7. Agustsson T, Rydén M, Hoffstedt J, Harmelen V, Dicker A, Laurencikiene J, et al. Mechanism of increased lipolysis in cancer cachexia. Cancer Res 2007; 67: 5531–5537. [DOI] [PubMed] [Google Scholar]
- 8. Kir S, White JP, Kleiner S, Kazak L, Cohen P, Baracos VE, et al. Tumour‐derived PTH‐related protein triggers adipose tissue browning and cancer cachexia. Nature 2014; 000: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fearon KC. The mechanisms and treatment of weight loss in cancer. Proc Nutr Soc 1992; 51: 251–265. [DOI] [PubMed] [Google Scholar]
- 10. Das SK, Eder S, Schauer S, Diwoky C, Temmel H, Guertl B, Gorkiewicz G, et al. Adipose triglyceride lipase contributes to cancer‐associated cachexia. Science 2011; 333: 233–238 (2011). [DOI] [PubMed] [Google Scholar]
- 11. Fearon KC Cancer cachexia and fat–muscle physiology. N Engl J Med 2011; 365: 565–567. [DOI] [PubMed] [Google Scholar]
- 12. Inui A. Cancer anorexia–cachexia syndrome: current issues. CA Cancer J Clin 2002; 52: 72–91. [DOI] [PubMed] [Google Scholar]
- 13. Fearon K, Arends J, Baracos V Understanding the mechanisms and treatment options in cancer cachexia. Nature Rev Clin Oncol 2013; 10: 90–99. [DOI] [PubMed] [Google Scholar]
- 14. Efferth T, Li PC, Konkimalla VS, Kaina B. From traditional Chinese medicine to rational cancer therapy. Trends Mol Med 2007; 13: 353–361. [DOI] [PubMed] [Google Scholar]
- 15. Mirza KA, Pereira SL, Edens NK, Tisdale MJ. Attenuation of muscle wasting in murine C2C12 myotubes by epigallocatechin‐3‐gallate. J Cachexia Sarcopenia Muscle 2014; 5: 339–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang H, Lai YJ, Chan YL, Li TL, Wu CJ. Epigallocatechin‐3‐gallate effectively attenuates skeletal muscle atrophy caused by cancer cachexia. Cancer Lett 2011; 305: 40–49. [DOI] [PubMed] [Google Scholar]
- 17. Yang QJ, Wan LL, Zhou ZY, Li Y, Yu Q, Liu LY, et al. Parthenolide from Parthenium integrifolium reduces tumor burden and alleviate cachexia symptoms in the murine CT‐26 model of colorectal carcinoma. Phytomedicine 2013; 20: 992–998. [DOI] [PubMed] [Google Scholar]
- 18. Qian EW, Ge DT, Kong SK. Salidroside protects human erythrocytes against hydrogen peroxide‐induced apoptosis. J Nat Prod 2012; 5: 531–537. [DOI] [PubMed] [Google Scholar]
- 19. Jin HJ, Pei L, Shu XG, Yang X, Yan TH, Wu Y, et al. Therapeutic intervention of learning and memory decays by salidroside stimulation of neurogenesis in aging. Mol Neurobiol 2014.(publish online) [DOI] [PubMed] [Google Scholar]
- 20. Zhang L, Yu HX, Zhao XC, Lin XF, Tan C, Cao G, et al. Neuroprotective effects of salidroside against beta‐amyloid‐induced oxidative stress in SH‐SY5Y human neuroblastoma cells. Neurochem Int 2010; 57: 547–555. [DOI] [PubMed] [Google Scholar]
- 21. Zhang J, Zhen YF, Ci Ren PB, Song LG, Kong WN, Shao TM, et al. Salidroside attenuates beta amyloid‐induced cognitive deficits via modulating oxidative stress and inflammatory mediators in rat hippocampus. Behav Brain Res 2013; 244: 70–81. [DOI] [PubMed] [Google Scholar]
- 22. Risson V, Mazelin L, Roceri M, Sanchez H, Moncollin V, Corneloup C, et al. Muscle inactivation of mTOR causes metabolic and dystrophin defects leading to severe myopathy. J Cell Biol 2009; 187: 859–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, et al. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo . Nat Cell Biol 2001; 3: 1014–1019. [DOI] [PubMed] [Google Scholar]
- 24. Eley HL, Russell ST, Tisdale MJ. Mechanism of attenuation of muscle protein degradation induced by tumor necrosis factor‐α and angiotensin II by β‐hydroxy‐β‐methylbutyrate. Am J Physiol Endocrinol Metab 2008; 295: E1417–E1426. [DOI] [PubMed] [Google Scholar]
- 25. Fearon KC, Glass DJ, Guttridge DC. Cancer cachexia: mediators, signaling, and metabolic pathways. Cell Metab 2012; 16: 153–166. [DOI] [PubMed] [Google Scholar]
- 26. Tisdale MJ. Cachexia in cancer patients. Nature 2002; 6: 862–871. [DOI] [PubMed] [Google Scholar]
- 27. Ovesen L, Allingstrup L, Hannibal J, Mortensen EL, Hansen OP. Effect of dietary counseling on food intake, body weight, response rate, survival, and quality of life in cancer patients undergoing chemotherapy: a prospective, randomized study. J Clin Oncol 1993; 1: 2043–2049. [DOI] [PubMed] [Google Scholar]
- 28. Tisdale MJ. Mechanisms of cancer cachexia. Physiol Rev 2009; 89: 381–410. [DOI] [PubMed] [Google Scholar]
- 29. Rosenberg B. Fundamental studies with cisplatin. Cancer 1985; 55: 2303–2316. [DOI] [PubMed] [Google Scholar]
- 30. Dy GK, Adjei AA. Understanding, recognizing, and managing toxicities of targeted anticancer therapies. CA Cancer J Clin 2013; 63: 249–279. [DOI] [PubMed] [Google Scholar]
- 31. Deblon N, Bourgoin L, Veyrat‐Durebex C, Peyrou M, Vinciguerra M, Caillon A, et al. Chronic mTOR inhibition by rapamycin induces muscle insulin resistance despite weight loss in rats. Brit J Pharmacol 2012; 165: 2325–2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement 1. Histopathological changes of kidney of each group in the combined chemotherapies in CT‐26 (Left) and LLC models (Right), respectively. Tissues of kidney from mice of each group were fixed in 4% polyformaldehyde, paraffin‐embedded, and then 4‐µm‐thick sections were stained with hematoxylin/eosin (HE). The right panel of each CAC model depicted higher magnifications of them
Supporting info item


