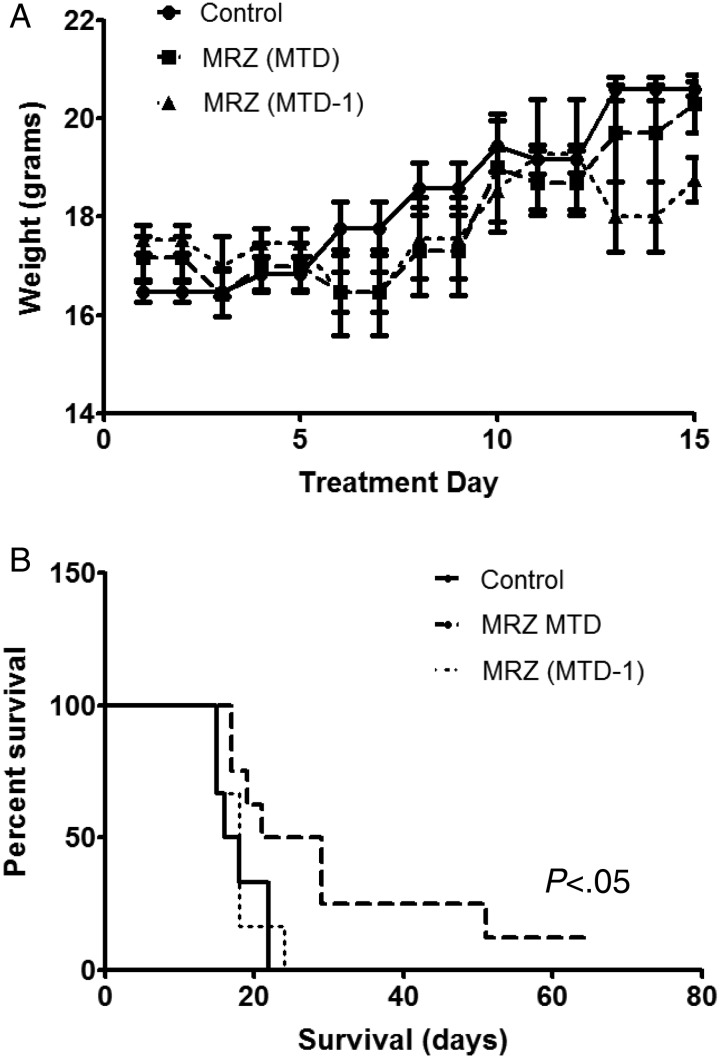
Fig. 5.
Marizomib prolonged animal survival in an orthotopic mouse glioma xenograft model. (A) Control vehicle or marizomib was administered twice weekly for 2.5 weeks (on days 1, 4, 8, 11, and 15) into the tail vein of athymic BALB/c nu/nu mice. There was no statistical difference in the weight of animals among the vehicle control, maximum tolerated dose group (MTD: 200 µg/kg) and lower dose group (MTD-1: 150 µg/kg). (B) Kaplan-Meier survival probability plots of tumor-bearing mice in vehicle or marizomib treatment groups (n = 6–8), using the log-rank method to test for a difference between groups.