Figure 4. RING dimerization and the interaction with ubiquitin.
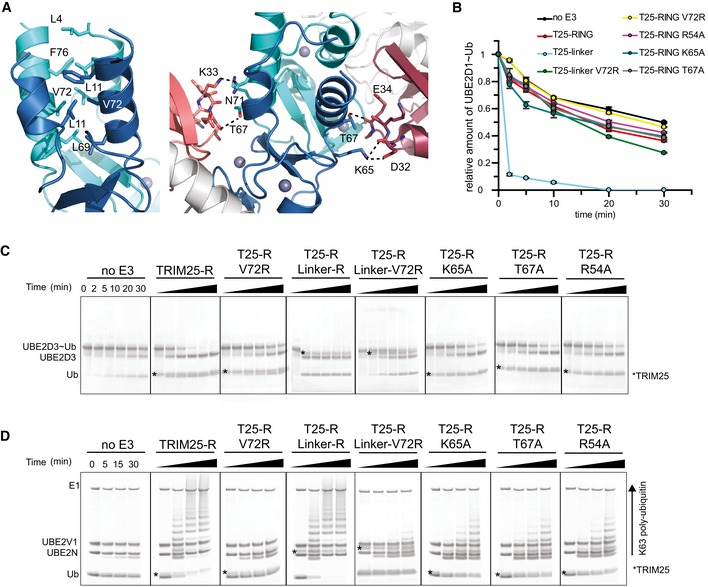
- Close‐up of the TRIM25 RING dimer interface highlighting the hydrophobic interactions made between the four α‐helices (left). Close‐up of the interface between each RING monomer and the proximal ubiquitin (right).
- UBE2D1˜UbAtto discharge assays with TRIM25 wild‐type RING, the fused RING constructs and different mutants important for dimerization or the interaction with ubiquitin. Time point zero for the T25‐R Linker and T25‐R Linker V72R samples was taken before the addition of E3 as discharge is very fast. The loss of UBE2D1˜UbAtto is plotted as the average of experimental duplicates (± s.d.).
- UBE2D3˜Ub discharge assays with the same mutants as in (B), stained with InstantBlue.
- K63 poly‐ubiquitination assays using UBE2N/UBE2V1. The asterisk indicates the band for the TRIM construct.
Source data are available online for this figure.
