Figure 5. Stabilization of the closed E2~Ub conformation.
-
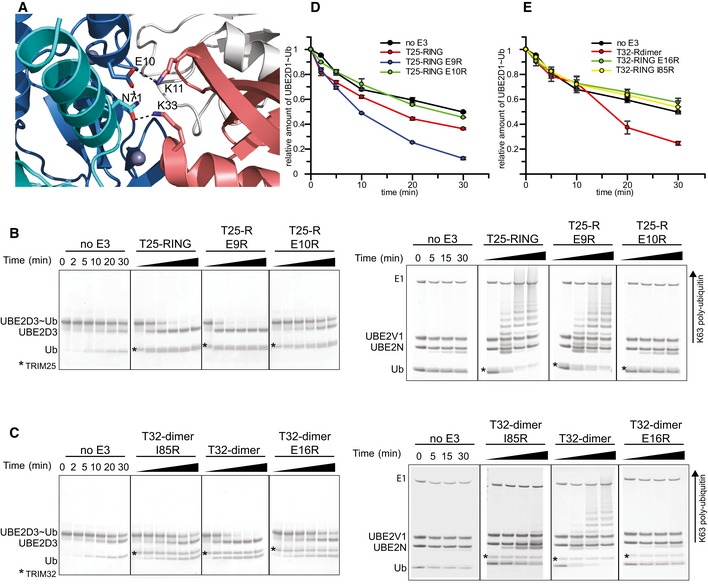
ARole of E10 in the RING of TRIM25 (blue) in stabilizing the closed E2˜Ub conformation by contacting K11 from the proximal ubiquitin (salmon) and N71 of the opposite RING (cyan) which in turn contacts K33 of the same ubiquitin.
-
BE2˜Ub discharge and K63 poly‐ubiquitination assays to test the role of E9 and E10 in TRIM25 activity. Substitution of Glu9 with Arg has no significant effect on activity, whereas the E10R mutation almost completely abolishes catalytic activity.
-
CDischarge and K63 poly‐ubiquitination assays to test the role of the equivalent residue E16 in TRIM32 and the role of I85R. Mutation of E16R abolishes catalytic activity indicating that the role of the glutamate is conserved.
-
D, EQuantification of UBE2D1˜UbAtto discharge assays. The loss of E2˜Ub is plotted as the average of experimental duplicates (± s.d.).
Source data are available online for this figure.
