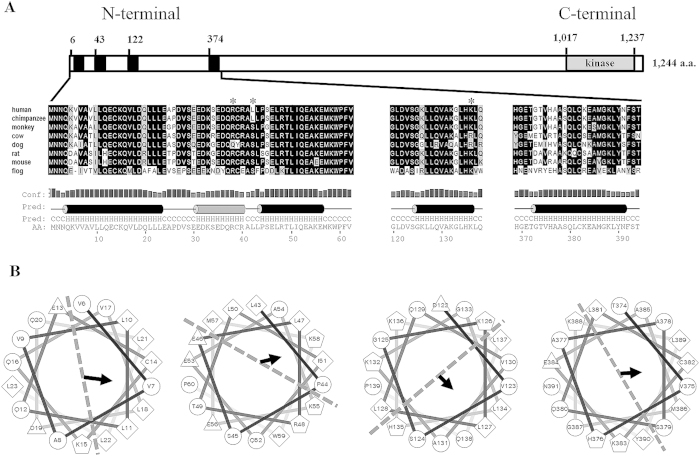
Figure 1. Conserved α-helical structures within ALPK1 N-terminal domain.
(A) An enlarged view of ALPK1 N-terminal region and cross-species comparison of N-terminal orthologues (asterisks denote highly conserved amino acid residues that have predicted noncovalent properties with myosin IIA); (B) N-terminal-conserved α-helical-rich regions of human ALPK1 protein can potentially fold into an amphipathic helical structure, well-suited for vesicular transport and curvature fusion sensors. Here, four putative amphipathic α-helical structures are shown that demonstrated partitions of hydrophobic, nonpolar, negative, and positive residues represented as diamonds, circle, triangles, and pentagons, respectively. The area separated by dotted line illustrates the amphipathic properties and the arrow indicates the hydrophobic moment and direction.