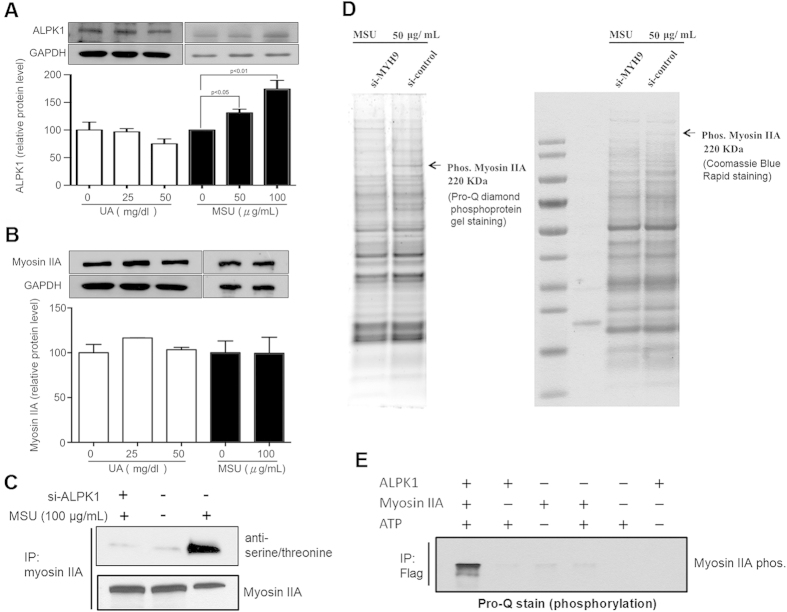
Figure 4. MSU-induced phosphorylation of myosin IIA is ALPK1 dependent.
(A) After 16-h treatments with uric acid (0, 25, and 50 mg/dl) or MSU crystals (0, 50, and 100 μg/mL), uric acid did not increase the protein expression of ALPK1, however MSU did (p < 0.05). (B) Uric acid and MSU did not affect myosin IIA expression. (C) Both MSU treatment and ALPK1 increased myosin IIA phosphorylation (lane 3) in THP-1 cells using antiphospho-serine/threonine antibody. (D) Phosphorylated myosin IIA was confirmed at the 220-kDa position, from comparing the depletion and no depletion of myosin IIA by MYH9 siRNA, on the Pro-Q staining SDS-PAGE gel (left) and protein-specific Coomassie Blue staining gel (right). A control without MSU stimulus is shown in Fig. S3. (E) Without MSU stimulus, the phosphorylation of myosin IIA is shown to require ALPK1 and ATP (lane 1) on the Pro-Q staining assay. The weak myosin IIA bands for example in second lane that has no myosin were trace myosin IIA bound to ALPK1 FL and Nt1, refer to Fig. 3C, from purification that was co-eluted.