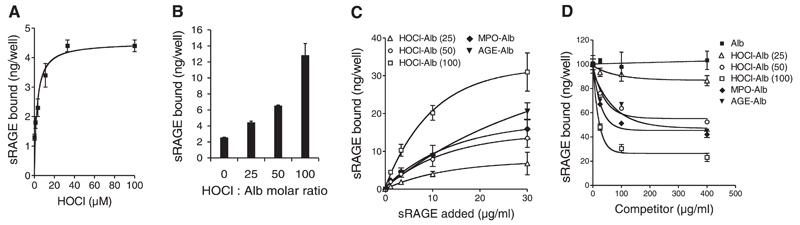
Figure 2.
sRAGE binding to HOCl-modified albumin. A) Albumin was immobilized onto wells of plastic dishes and modified by indicated HOCl-concentrations. After washing and blocking of excess binding sites with native albumin, 125I-sRAGE (3 µg/ml) was added to coated wells. Subsequently, wells were washed and radioactivity was counted. B) Native albumin (Alb) and freshly prepared HOCl-albumin (oxidant:protein molar ratio of 25:1, 50:1, and 100:1) was immobilized onto wells of plastic dishes. After washing and blocking of excess binding sites with native albumin, 125I-sRAGE (3 µg/ml) was added to coated wells. Subsequently, wells were washed, and radioactivity was counted. C) Albumin (Alb), AGE-Alb, HOCl-Alb (oxidant:protein molar ratio of 25:1, 50:1, and 100:1) and MPO-Alb (modified by the MPO-H2O2-chloride system; oxidant:protein molar ratio of 50:1) were immobilized onto wells of plastic dishes. After washing and blocking of unspecific binding sites, indicated concentrations of 125I-sRAGE (1, 3, 10, and 30 µg/ml) were added to coated wells. Subsequently, wells were washed and radioactivity was counted. Binding of 125I-sRAGE to control albumin-coated wells (nonspecific binding) was subtracted from binding values to modified-albumin to calculate specific binding. Only specific data are shown. D) Dose-response curves for 125I-sRAGE (5 µg/ml) binding inhibition to AGE-BSA-coated microtiter wells by albumin (Alb), AGE-Alb, HOCl-Alb (oxidant:protein molar ratio of 25:1, 50:1, and 100:1) and MPO-Alb (modified by the MPO-H2O2-chloride system; H2O2:Alb molar ratio of 50:1). Results represent mean ± sd (n=3) of one experiment out of four.