Abstract
Increases in our understanding of the molecular control of circadian rhythms and subsequent signaling pathways has allowed for new therapeutic drug targets to be identified as well as for a better understanding of how to more efficaciously and safely utilize current drugs. Here, we review recent advances in targeting components of the molecular clock in mammals for the development of novel therapeutics as well as describe the impact of the circadian rhythm on drug efficacy and toxicity.
Keywords: Circadian rhythm, Drug discovery
Most, if not all light-sensitive organisms, including mammals, possess a master circadian clock that regulates physiological and behavioral processes in alignment with the 24-h day. Recent advances in understanding the molecular control of circadian rhythms and subsequent signaling pathways has allowed for new therapeutic targets to be identified as well as for a better understanding of how to more efficaciously utilize current drugs. In addition to playing a key role in normal physiology and behavior, aberrations in the circadian rhythm are associated with the pathophysiology of diseases including diabetes, cardiovascular disease, psychological disorders and various autoimmune diseases/inflammation.1–5 Additionally, the potency and efficacy of many drugs is associated with the circadian rhythmicity of expression of their molecular targets and cellular biochemical signals.4 A significant fraction of genes are expressed in a circadian fashion6 including many drug targets and drug metabolizing enzymes yielding potential differences in efficacy/side effects based on time of day administration. Applying the knowledge of circadian function and regulation to the relevance of disease has enabled a chronotherapy approach in the timing of administration of conventional drugs in order to synchronize the rhythms in disease activity with the efficacy of the drug. Furthermore, recent advances in targeting the protein components involved in maintenance of the core circadian rhythm have allowed us to examine pharmacological alteration of the core rhythm to examine potential utility for treatment of disease.
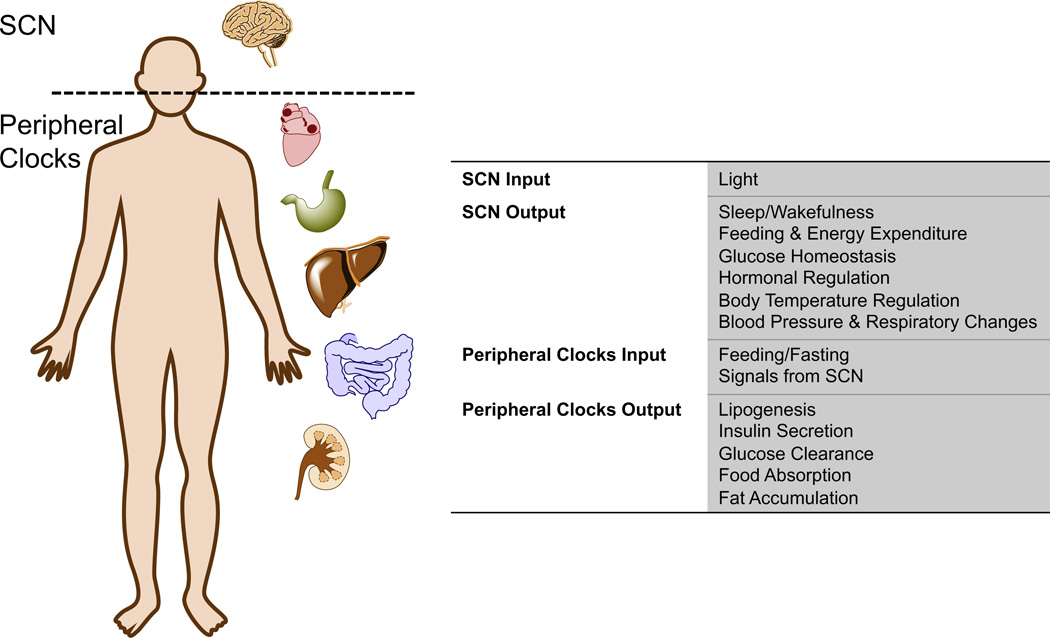
Circadian rhythms influence most, if not all of mammalian physiology. Optimizing and coordinating metabolic processes, cellular functions and the organism’s behavior including the sleep-wake cycle can be viewed as the main tasks of the circadian timing system (CTS).2 The CTS is hierarchical in structure with the master pacemaker located in the superchiasmatic nucleus (SCN) of the hypothalamus. This master pacemaker coordinates countless peripheral clocks within various tissue and cell types. In general, the molecular makeup of oscillators within the SCN and the periphery are very similar, with the main difference being how they are synchronized and influenced by various signals. The SCN is entrained by light received by the retina while the peripheral oscillators are often adjusted by chemical signals or by feeding2,7– 9 (Fig. 1).
Figure 1.
Circadian clocks exist both in the central nervous system and in the peripheral tissues. The figure illustrates the master clock (suprachiasmatic nucleus (SCN)) is via light received by the retina. The peripheral clocks of many organs are also illustrated and can be entrained by signals from the SCN as well as other signals such as nutrient availability.
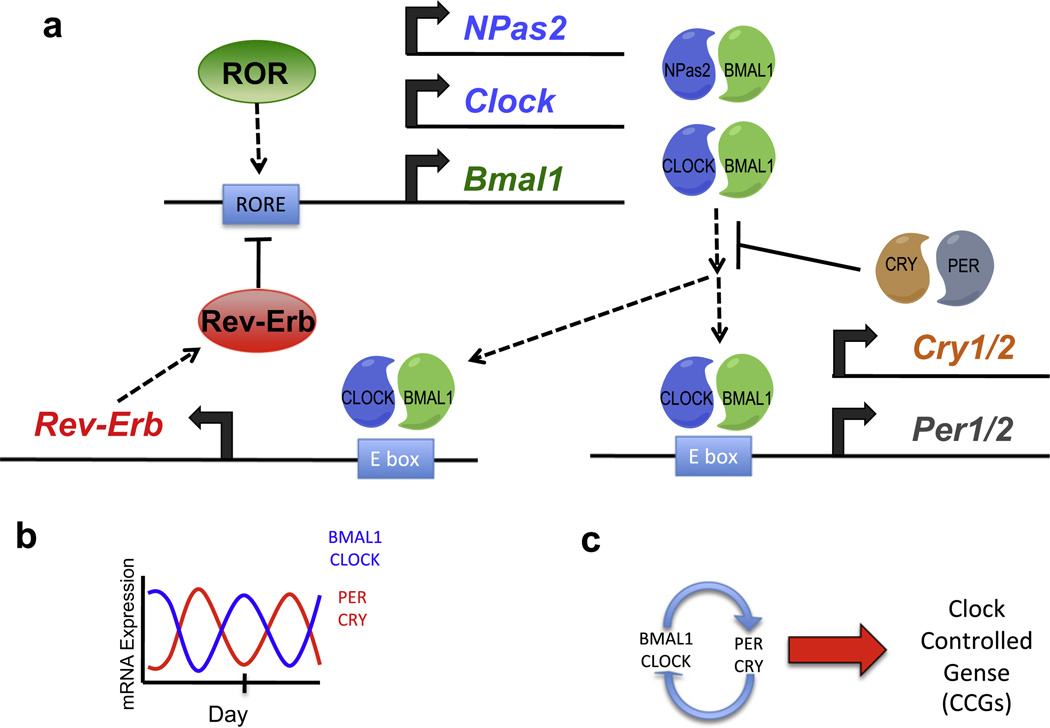
Feedback regulation on a transcriptional and posttranscriptional level creates and maintains the circadian oscillations in the context of a single cell. Heterodimers of transcription factors, either CLOCK or NPAS2 with BMAL1 bind to DNA E-box elements within enhancer and promoter regions of the Cryptochrome (Cry) and Period (Per) genes and stimulate their transcription. CRY and PER protein levels accumulate to form complexes that inhibit the BMAL1-CLOCK/NPAS2 heterodimers, leading to the loss of activation of Cry and Per genes, and a reduction of CRY and PER protein levels which leads to the next transcriptional cycling event.2,10,11 Additionally, BMAL1–CLOCK/NPAS2 heterodimers also drive the circadian expression of REV-ERB, an nuclear receptor that represses both Bmal1 and Clock gene transcription by direct binding to their promoters12–14 (Fig. 2). An additional nuclear receptor, the retinoic acid receptor-related orphan receptor (ROR) also plays an important role activating transcription of the Bmal1 gene by binding to the identical DNA response element recognized by REV-ERB.15–17 Other proteins such as the WD40 protein, NONO DNA-binding protein, the WDR5 histone methyl transferase binding platform, and casein kinases (CK1) also play an important role in modulation of the feedback loop ensuring proper circadian function.2,18 This self-sustained, cell autonomous circadian oscillator is maintained in most, if not all mammalian cells19 and drives physiological alterations by controlling genes that are responsive to components of the oscillator—clock controlled genes (CCGs).
Figure 2.
The mammalian circadian clock. (a) The mechanism of the mammalian molecular clock illustrating the feedback loop that maintains 24-h oscillations of BMAL1-CLOCK/NPAS2 and PER-CRY expression. (b) Illustration of antiphase circadian oscillations of BMAL1-CLOCK/NPAS2 and PER-CRY expression. (c) Schematic illustrating the relationship between the core clock and clock-controlled genes.
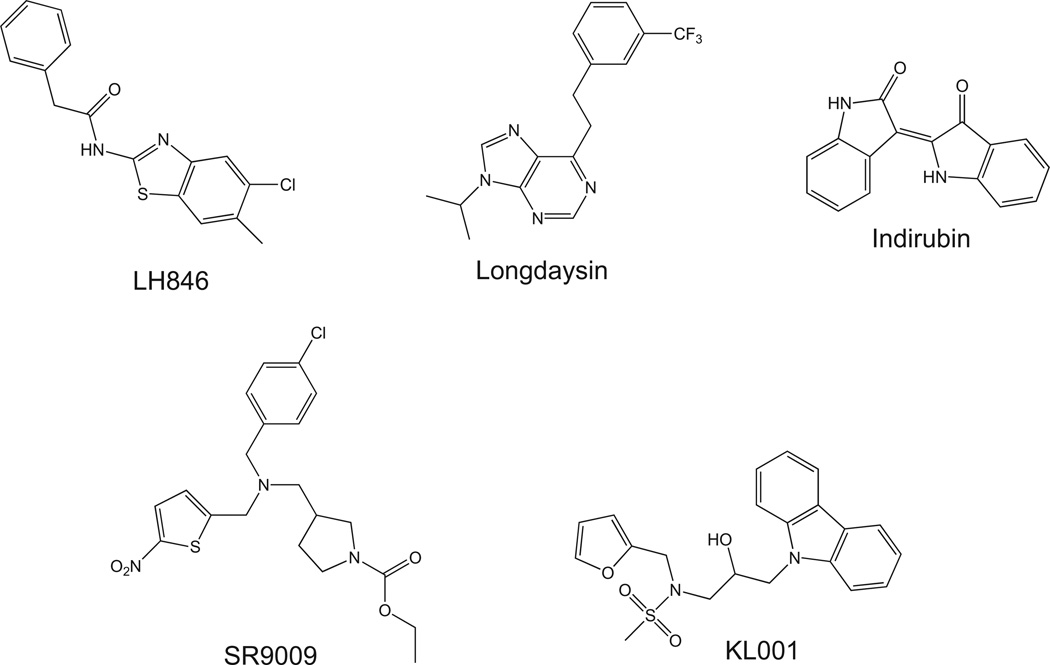
Some components of the mammalian clock described above are clearly ‘druggable’ and very recent studies have demonstrated the effects of modulating the activity of these targets on circadian physiology as well as pathological states. The nuclear receptor REV-ERB was recently deorphanized and heme was identified as the physiological ligand20,21 and these studies clearly suggested that synthetic compounds could directly target this nuclear receptor. Our group recently developed synthetic agonists for REV-ERB, SR9009, that demonstrated the ability to modulate circadian behavior as well as metabolism3 (Fig. 3). Administration of REV-ERB agonists in mice resulted in alterations in the expression of clock genes both in the SCN and the periphery (liver, adipose and skeletal muscle). Circadian wheel running behavior was altered and interestingly, mice displayed increased energy expenditure with no increase in locomotion or food intake. Additionally, when administered to diet-induced obese mice, REV-ERB agonist SR9009 induced significant weight loss (fat mass) and reduced plasma triglycerides and cholesterol levels. Clearly, these data suggest that targeting the clock may hold utility in the treatment of metabolic disorders. A number of synthetic ligands that target the ROR nuclear receptors have also been recently identified; however, their ability to modulate the circadian rhythm has not yet been examined.22–27
Figure 3.
Synthetic compounds that modulate the clock. Chemical structures of several compounds that alter the circadian rhythm by targeting the core clock are shown. LH846 and longdaysin are CK1 inhibitors while indirubin is a GSK3β inhibitor. SR9009 is a REV-ERB agonist and KL001 is an activator of CRY.
Small molecule activators of the CRY proteins were recently identified using a cell-based circadian phenotypic screen.28 KL001 interacts specifically with CRY and prevents its ubiquitin-dependent degradation leading to lengthening of the circadian period in cell-based models28 (Fig. 3). Additionally, KL001 modulation of CRY stability led to inhibition of glucagon-induced gluconeogenesis in primary hepatocytes providing further evidence that targeting the clock may hold utility in the treatment of metabolic disorders. No studies with KL001 or related compounds have been performed in vivo yet.
The CK1 family of kinases has been implicated in the phosphorylation of PER proteins and modulation of their activity. CK1δ has been implicated as the dominant isozyme required for regulation of the circadian period based on studies with isozyme selective CK1 inhibitors.29 Longdaysin, an inhibitor of CK1α, CK1δ and extracellular signal-regulated kinase 2 (ERK2), was identified in a screen of compounds based on their ability to lengthen the circadian period and this compound appears to function via stabilization of PER130 (Fig. 3). Administration of longdaysin to zebrafish resulted in a lengthened circadian period. Other CK1δ inhibitors such as LH846 have also been identified and have the ability to lengthen the circadian period in cell based models31 (Fig. 3). In contrast to the CK1δ inhibitors, inhibitors of glycogen synthase kinase β (GSK3β), such as indirubin, appear to shorten the circadian period32 (Fig. 3). GSK3β substrates include several components of the clock including REV-ERB, BMAL1, CLOCK and CRY2, thus it is not clear exactly how these compounds mechanistically alter the clock. Clearly, there appear to be multiple opportunities to develop small molecule modulators of clock components and employ a chemical biology approach to determine the potential therapeutic utility of such compounds.
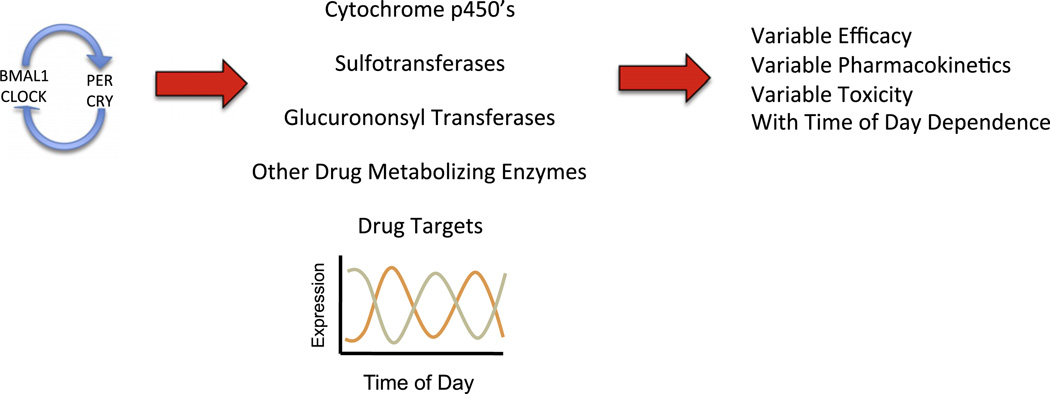
Variations in gene expression due to circadian changes often have effects on the absorption, distribution, metabolism and elimination of drugs2 (Fig. 4). These effects result from the rhythmic changes in liver metabolism, membrane viscosity, blood flow in the periphery as well as through organs, renal filtration rates, and various other rhythmic physiological properties. Clearly, efficacy and tolerability of a drug can be severely impacted by circadian pharmacokinetics and pharmacodynamics.
Figure 4.
Schematic illustrating the role of the clock in regulation of oscillations in various enzymes and drug target expression levels that lead to variability in drug efficacy, pharmacokinetics and toxicity with regard to the time of day administration of a drug.
One of the most extensively studied examples of time-dependent dosing on drug effect and toxicity is glucocorticoid receptor (GR) agonists. GR is a ubiquitously expressed, glucocorticoid-activated member of the nuclear receptor superfamily of transcription factors and is involved in the regulation of development, metabolism, and immune response. The natural ligands for GR are glucocorticoids, predominantly cortisol, and are synthesized from cholesterol precursors in the adrenal cortex under tight regulation from hypothalamo-pituitary axis. Circulating levels of glucocorticoids fluctuate in a circadian manner, with the highest levels observed in the morning and the lowest levels in the late evening in diurnal animals. Two decades ago, Beam et al. described a relationship between time of day of administration of a synthetic GR agonist, prednisone, and relief of nocturnal worsening of asthma symptoms.33 They speculated that the worsening of asthmatic symptoms at night was related to the corticosteroid-sensitive circadian localization of eosinophils to the lungs.33 Subjects were treated with oral prednisone at one of several time points (0800, 1500, and 2000 h) for six weeks and monitored by bronchoscopy, spirometry, and blood eosinophil levels. Interestingly the timing of prednisone was absolutely critical for the relief of the nocturnal worsening of the symptoms and the 1500 h administration of prednisone was effective while dosing at either 0800 h or 2000 h was ineffective.33
More recently GR function has been shown to be closely linked to the mammalian clock. The CLOCK/BMAL1 complex directly interacts and enzymatically targets GR, thereby regulating the action of its ligands in peripheral tissues.34 It was suggested that the CLOCK/BMAL1 heterodimer acetylates the lysine-rich area of the GR hinge region and suppresses transcriptional activity regulated by GR, indicating that components of the circadian clock can modulate the effects of glucocorticoids on immune functions in various tissues and organs.34 Studies in Per2/Cry1 knockout mice, which lack a circadian rhythm, displayed defective pituitary and adrenal regulation. Interestingly, tissue transplantation in these mice led to a rescued glucocorticoid rhythm under LD conditions.35 Most recently it was demonstrated that CRY1 and CRY2 directly interact with GR and modulate the ligand-dependent activity of the receptor suggesting another direct link between GR function and a core component of the mammalian clock.36
Many anticancer therapeutics have been shown to exhibit differing effects and toxicity based on administration time in rodents.37,38 Gorbacheva et al. showed the clear relationship between the chemotherapeutic agent, cyclophosphamide, and functional timing of the CLOCK/BMAL1 circadian components.37 Several different mutant mouse models were used to explore the molecular link between in vivo cyclophosphamide drug toxicity and circadian function, when compared to control mice treated with the same drug. It was observed that animals treated at the dark:light transition were more sensitive to drug toxicity than those treated at the light:dark transition; the increased drug sensitivity also appeared to correlate with survival of the animals37 Clock mutants and Bmal1−/− mice also showed a severe reduction in body weight following cyclophosphamide treatment, whereas the control mice maintained a stable body weight following injections. These findings suggested that when CLOCK/BMAL1 activity is deficient mice display a higher sensitivity to the chemotherapeutic drug. In addition, this and several other groups hypothesized that the circadian regulation of drug sensitivity may be further controlled by peripheral clocks regulating the rhythmic activity of drug-metabolizing enzymes, and not solely through the CLOCK/ BMAL1 transactivation pathway in the SCN.2,37,39
Since many of the components of the detoxification system have circadian mRNA expression, it makes sense that toxicity of certain drugs depends on the time of dosing. Members of cytochrome p450 (CYP) family of drug-metabolizing enzymes are highly expressed in the liver, kidney, intestine, and other organs. These enzymes often have oxidase, reductase, or hydrolase activities that aid in the removal of toxins.2 Additionally, conjugating enzymes, like sulfonotransferases, modify lipophillic components of drugs and allow for excretion into bile or urine. Most enzymes within the detoxification pathway have been shown to display circadian fluctuation throughout the day that likely contribute to time-dependent efficacy of specific drugs.2 An additional component of the molecular basis of detoxification involves several nuclear receptors including constitutive androstane receptor (CAR), pregnane X receptor (PXR), peroxisome proliferator-activated receptor (PPAR), liver X receptor (LXR), farnesoid X receptor (FXR), and hepatocyte nuclear factor (HNF4), and other transcriptional regulatory proteins like the aryl hydrocarbon receptor (AHR).2,40 Several of these proteins regulate the detoxification system in a circadian manner as they either accumulate in a circadian manner or themselves display a circadian rhythm.2 Various studies suggest that the circadian expression of many nuclear receptors may shed light on how circadian rhythms are linked to disease and also how to effectively treat an individual.8,40–42 Iwata et al. using the broad-spectrum anti-cancer drug gemcitabine (GEM) recently described an example of this.42 Several studies revealed that standard weekly treatments of GEM often led to unpleasant side effects including myelosuppression, nausea, vomiting, alopecia, and varying hematologic toxicity in patients.18,39,42 Patients were randomly divided into two groups, either receiving treatment at 9 am or 3 pm, and the difference between hematologic toxicity between the groups over a two-week period.42 In addition to determining changes in white blood cell counts, red blood cell, hematocrit, hemoglobin and other serum levels were also assessed. This group was able to show that dosing with GEM earlier in the day alleviated hematologic toxicity. This is likely due to the rapid metabolism of GEM in the liver, kidneys, and plasma by cytidine deaminase, which has circadian expression in humans and peaks during the early active phase.42 Interestingly, studies of GEM in mice also show that circadian expression of BAX and BCL-2 may also contribute to the tolerability of this drug, since it targets S-phase cells and promotes apoptosis.42
In a recent study, Hamdan et al. found that the Abcg2 intestinal gene expression in mice is under the circadian control of the ATF4-CLOCK pathway and further investigated how fluctuations in gene expression affected oral absorption of a classic substrate of the breast cancer resistance protein (BCRP) in vivo.43 The well-characterized BCRP substrate sulfasalazine was orally administered to Abcg2 knockout demonstrating a 10-fold increase in accumulation than in wildtype mice. Interestingly, in the wildtype mice, intestinal accumulation of sulfasalazine displayed a circadian rhythm antiphase to that of BCRP expression.43 It has been speculated that BCRP expression in the mouse intestine is regulated by peripheral clocks and regulates the rate of intestinal drug absorption. It should also be mentioned that alternative routes of drug administration, including oral, cutaneous/transdermal, and intravenous, have differences in drug absorption rates influenced by circadian rhythms.4 It has been demonstrated previously that local anesthetic agents have varying penetration rates through the skin in a circadian time-dependent manner.4,44,45 Therefore, drug formulation, route of administration and circadian rhythms influences should all be taken into account when treating a disease.4,44,45 Taken together, the studies mentioned thus far suggest a molecular link between circadian rhythms and drug pharmacology that should be exploited in future therapeutic studies.
Another consideration when thinking about the chronopharmacology of a drug or compound is the delivery of treatment to a patient based on the dynamic disease-specific pathology and the changes in the pharmacology of that particular drug, often referred to as chronotherapeutics.2,40,46 This relatively new discipline of medical treatment allows for the consideration of a patient’s biological rhythm, changes in the severity of a disease state during the day, and the synchronizing of dosing and delivery of a particular drug to allow for the optimal efficacy in the patient.46,47 Many studies have shown that cardiovascular related events, such as myocardial infarction and stroke, often occur in the early morning due to circadian changes regulating blood pressure, heart rate, vasodilating hormones, etc.47,48 Dihydropyridine calcium channel blockers, such as Norvasc, have been shown that time of day dosing, particularly evening, is the most advantageous for efficacy of the treatment and normalizing changes in blood pressure rhythms to reduce early morning cardiac events.47,48 ACE inhibitors, AT-receptor blockers, diuretics, and other antihypertensives have also been studied to determine circadian-based time of day dosing for the most effective treatment.
While many people acknowledge that targeting diseases with specific time-of-day dosing of drugs based on circadian rhythms is beneficial for patients in increasing efficacy while reducing side effects, it is often overlooked that disruption of circadian rhythms can lead to disease. Disruption of circadian rhythms is often observed in individuals involved with shift-work or sudden changes in time schedule from traveling (jet-lag), and often contribute to fluctuations in sleep patterns, behaviors, and metabolic patterns. In the case of jet-lag, circadian disruption is temporary but often leads to feelings of general discomfort, irritability, depression, gastrointestinal upset, and a decreased mental and physical performance.49,50 Similar to individuals with jet-lag, shift workers tend to have circadian disruption of the master and peripheral clocks, leading to an increased incidence of gastrointestinal, cardiovascular, and metabolic diseases, as well as menstrual problems in women, and increased cancer risks.49 It should also be noted that even low-intensity light exposure at night can disrupt the clock in humans, which often alters temperature and hormonal rhythms and may lead to an increased disease propensity.49,51 An interesting study performed by Cheeseman et al. tested the effects of general anesthesia on time perception and circadian rhythm in honeybees.52 Honeybees have a remarkably similar genetic and functional molecular circadian network to mammals and this work demonstrated that isoflurane exposure, a general anesthetic, led to abnormal orienting behavior, alterations in foraging time, atypical circadian rhythms, and severely altered circadian gene expression markers. It was also demonstrated that the phase shifting caused by the use of isoflurane was in direct contrast with those caused by pulsed light. When isoflurane or pulsed light was administered during daytime, a strong phase delay was observed for the isoflurane treated bees but not the light-treated bees. Conversely, when administered at night, only the light treatment caused a phase delay in the bees.52 These results suggest a possible neurobiological mechanism that may be shifting the circadian clock in honeybees and can be extrapolated to circadian shifts and time perception in mammals following anesthesia administration. An important question to consider is whether it may be possible to target the circadian mechanisms in order to treat patients and help expedite recovery time following surgical procedures.52
It is clear that environmental cues drive the rhythmic expression of clock genes in mammals. Many physiological processes in humans are influenced by both the CTS of the master clock in the SCN and various peripheral clocks found in differing tissues. Recent studies have begun to decipher the molecular networks involved in the regulation and coordination of these clocks, and thus furthering the understanding of how circadian timing affects physiological processes and disease development. The latest advances in chronopharmacology are leading the way to unleash new opportunities for drug discovery and advance drug development. As mentioned previously, it has been shown that circadian rhythms influence how drugs are absorbed, distributed, metabolized, and eliminated from the body. Development of circadian-controlled release and/or delivery of current therapeutics may enhance treatment efficacy and control cell toxicity, especially in chemotherapeutic agents. Further research should be applied to optimizing novel drugs to synchronize with a patient’s circadian rhythm, in order to increase the benefit of treatment.53 Additionally, the development of drugs that act by specifically modulating the components of the circadian clock is another highly anticipated area of chronopharmacology.
References and notes
- 1.Rybak YE, McNeely HE, Mackenzie BE, Jain UR, Levitan RD. Compr. Psychiatry. 2007;48:562. doi: 10.1016/j.comppsych.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 2.Levi F, Schibler U. Annu. Rev. Pharmacol. Toxicol. 2007;47:593. doi: 10.1146/annurev.pharmtox.47.120505.105208. [DOI] [PubMed] [Google Scholar]
- 3.Solt LA, Wang Y, Banerjee S, Hughes T, Kojetin DJ, Lundasen T, Shin Y, Liu J, Cameron MD, Noel R, Yoo S-H, Takahashi JS, Butler AA, Kamenecka TM, Burris TP. Nature. 2012;485:62. doi: 10.1038/nature11030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ohdo S. Biol. Pharm. Bull. 2010;33:159. doi: 10.1248/bpb.33.159. [DOI] [PubMed] [Google Scholar]
- 5.Sprouse J. Expert Opin. Ther. Targets. 2004;8:25. doi: 10.1517/14728222.8.1.25. [DOI] [PubMed] [Google Scholar]
- 6.Yan J, Wang H, Liu Y, Shao C. PLoS Comput. Biol. 2008;4:e1000193. doi: 10.1371/journal.pcbi.1000193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dallmann R, Viola AU, Tarokh L, Cajochen C, Brown SA. Proc. Natl. Acad. U.S.A. 2012;109:2625. doi: 10.1073/pnas.1114410109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iurisci I, Filipski E, Sallam H, Harper F, Guettier C, Maire I, Hassan M, Iacobelli S, Levi F. Chronobiol. Int. 2009;26:1169. doi: 10.3109/07420520903209942. [DOI] [PubMed] [Google Scholar]
- 9.Langmesser S, Albrecht U. Chronobiol. Int. 2006;23:151. doi: 10.1080/07420520500464437. [DOI] [PubMed] [Google Scholar]
- 10.Albrecht U. J. Physiol. Paris. 2006;100:243. doi: 10.1016/j.jphysparis.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 11.Mohawk JA, Green CB, Takahashi JS. Annu. Rev. Neurosci. 2012;35:445. doi: 10.1146/annurev-neuro-060909-153128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guillaumond F, Dardente H, Giguere V, Cermakian N. J. Biol. Rhythms. 2005;20:391. doi: 10.1177/0748730405277232. [DOI] [PubMed] [Google Scholar]
- 13.Crumbley C, Burris TP. PLoS ONE. 2011;6:e17290. doi: 10.1371/journal.pone.0017290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Preitner N, Damiola F, Molina LL, Zakany J, Duboule D, Albrecht U, Schibler U. Cell. 2002;110:251. doi: 10.1016/s0092-8674(02)00825-5. [DOI] [PubMed] [Google Scholar]
- 15.Akashi M, Takumi T. Nat. Struct. Mol. Biol. 2005;12:441. doi: 10.1038/nsmb925. [DOI] [PubMed] [Google Scholar]
- 16.Burris TP. Mol. Endocrinol. 2008;22:1509. doi: 10.1210/me.2007-0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sato TK, Panda S, Miraglia LJ, Reyes TM, Rudic RD, McNamara P, Naik KA, Fitzgerald GA, Kay SA, Hogenesch JB. Neuron. 2004;43:527. doi: 10.1016/j.neuron.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 18.Levi F, Filipski E, Iurisci I, Li XM, Innominato P. Cold Spring Harb. Symp. Quant. Biol. 2007;72:465. doi: 10.1101/sqb.2007.72.030. [DOI] [PubMed] [Google Scholar]
- 19.Nagoshi E, Saini C, Bauer C, Laroche T, Naef F, Schibler U. Cell. 2004;119:693. doi: 10.1016/j.cell.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 20.Raghuram S, Stayrook KR, Huang P, Rogers PM, Nosie AK, McClure DB, Burris LL, Khorasanizadeh S, Burris TP, Rastinejad F. Nat. Struct. Mol. Biol. 2007;14:1207. doi: 10.1038/nsmb1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yin L, Wu N, Curtin JC, Qatanani M, Szwergold NR, Reid RA, Waitt GM, Parks DJ, Pearce KH, Wisely GB, Lazar MA. Science. 2007;318:1786. doi: 10.1126/science.1150179. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y, Kumar N, Nuhant P, Cameron MD, Istrate MA, Roush WR, Griffin PR, Burris TP. ACS Chem. Biol. 2010:1029. doi: 10.1021/cb100223d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumar N, Kojetin DJ, Solt LA, Kumar KG, Nuhant P, Duckett DR, Cameron MD, Butler AA, Roush WR, Griffin PR, Burris TP. ACS Chem. Biol. 2011;6:218. doi: 10.1021/cb1002762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Solt LA, Kumar N, Nuhant P, Wang YJ, Lauer JL, Liu J, Istrate MA, Kamenecka TM, Roush WR, Vidovic D, Schurer SC, Xu JH, Wagoner G, Drew PD, Griffin PR, Burris TP. Nature. 2011;472:491. doi: 10.1038/nature10075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumar N, Lyda B, Chang MR, Lauer JL, Solt LA, Burris TP, Kamenecka TM, Griffin PR. ACS Chem. Biol. 2012;7:672. doi: 10.1021/cb200496y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Solt LA, Kumar N, He Y, Kamenecka TM, Griffin PR, Burris TP. ACS Chem. Biol. 2012;7:1515. doi: 10.1021/cb3002649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huh JR, Leung MWL, Huang PX, Ryan DA, Krout MR, Malapaka RRV, Chow J, Manel N, Ciofani M, Kim SV, Cuesta A, Santori FR, Lafaille JJ, Xu HE, Gin DY, Rastinejad F, Littman DR. Nature. 2011;472:486. doi: 10.1038/nature09978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hirota T, Lee JW, John PC, Sawa M, Iwaisako K, Noguchi T, Pongsawakul PY, Sonntag T, Welsh DK, Brenner DA, Doyle FJ, 3rd, Schultz PG, Kay SA. Science. 2012;337:1094. doi: 10.1126/science.1223710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meng QJ, Maywood ES, Bechtold DA, Lu WQ, Li J, Gibbs JE, Dupre SM, Chesham JE, Rajamohan F, Knafels J, Sneed B, Zawadzke LE, Ohren JF, Walton KM, Wager TT, Hastings MH, Loudon AS. Proc. Natl. Acad. Sci. U.S.A. 2010;107:15240. doi: 10.1073/pnas.1005101107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hirota T, Lee JW, Lewis WG, Zhang EE, Breton G, Liu X, Garcia M, Peters EC, Etchegaray JP, Traver D, Schultz PG, Kay SA. PLoS Biol. 2010;8:e1000559. doi: 10.1371/journal.pbio.1000559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee JW, Hirota T, Peters EC, Garcia M, Gonzalez R, Cho CY, Wu X, Schultz PG, Kay SA. Angew. Chem. Int. Ed. 2011;50:10608. doi: 10.1002/anie.201103915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reischl S, Kramer A. FEBS Lett. 2011;585:1393. doi: 10.1016/j.febslet.2011.02.038. [DOI] [PubMed] [Google Scholar]
- 33.Beam WR, Weiner DE, Martin RJ. Am. Rev. Respir. Dis. 1992;146:1524. doi: 10.1164/ajrccm/146.6.1524. [DOI] [PubMed] [Google Scholar]
- 34.Nader N, Chrousos GP, Kino T. FASEB J. 2009;23:1572. doi: 10.1096/fj.08-117697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oster H, Damerow S, Kiessling S, Jakubcakova V, Abraham D, Tian J, Hoffmann MW, Eichele G. Cell Metab. 2006;4:163. doi: 10.1016/j.cmet.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 36.Lamia KA, Papp SJ, Yu RT, Barish GD, Uhlenhaut NH, Jonker JW, Downes M, Evans RM. Nature. 2011;480:552. doi: 10.1038/nature10700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gorbacheva VY, Kondratov RV, Zhang R, Cherukuri S, Gudkov AV, Takahashi JS, Antoch MP. Proc. Natl. Acad. Sci. U.S.A. 2005;102:3407. doi: 10.1073/pnas.0409897102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dibner C, Schibler U, Albrecht U. Annu. Rev. Physiol. 2010;72:517. doi: 10.1146/annurev-physiol-021909-135821. [DOI] [PubMed] [Google Scholar]
- 39.Levi F, Okyar A, Dulong S, Innominato PF, Clairambault J. Annu. Rev. Pharmacol. Toxicol. 2010;50:377. doi: 10.1146/annurev.pharmtox.48.113006.094626. [DOI] [PubMed] [Google Scholar]
- 40.Levi F, Focan C, Karaboue A, de la Valette V, Focan-Henrard D, Baron B, Kreutz F, Giacchetti S. Adv. Drug Deliv. Rev. 2007;59:1015. doi: 10.1016/j.addr.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 41.Yang XY, Downes M, Yu RT, Bookout AL, He WM, Straume M, Mangelsdorf DJ, Evans RM. Cell. 2006;126:801. doi: 10.1016/j.cell.2006.06.050. [DOI] [PubMed] [Google Scholar]
- 42.Iwata K, Aizawa K, Sakai S, Jingami S, Fukunaga E, Yoshida M, Hamada A, Saito H. Biol. Pharm. Bull. 2011;34:1765. doi: 10.1248/bpb.34.1765. [DOI] [PubMed] [Google Scholar]
- 43.Hamdan AM, Koyanagi S, Wada E, Kusunose N, Murakami Y, Matsunaga N, Ohdo S. J. Biol. Chem. 2012;287:17224. doi: 10.1074/jbc.M111.333377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martin C, Sastre B, Mallet MN, Bruguerolle B, Brun JP, De Micco P, Gouin F. Antimicrob. Agents Chemother. 1991;35:2602. doi: 10.1128/aac.35.12.2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bruguerolle B, Giaufre E, Prat M. Chronobiol. Int. 1991;8:277. doi: 10.3109/07420529109063932. [DOI] [PubMed] [Google Scholar]
- 46.Smolensky MH, Peppas NA. Adv. Drug Deliv. Rev. 2007;59:828. doi: 10.1016/j.addr.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 47.Lin SY, Kawashima YJ. Control Release. 2012;157:331. doi: 10.1016/j.jconrel.2011.09.065. [DOI] [PubMed] [Google Scholar]
- 48.Lemmer B. ChronoPhysiol. Ther. 2012;2:9. [Google Scholar]
- 49.Escobar C, Salgado-Delgado R, Gonzalez-Guerra E, Osorio AT, Angeles-Castellanos M, Buijs RM. Sleep Disorders. 2011:2011. doi: 10.1155/2011/964510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sukumaran S, Almon RR, DuBois DC, Jusko WJ. Adv. Drug Deliv. Rev. 2010;62:904. doi: 10.1016/j.addr.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mavanji V, Billington CJ, Kotz CM, Teske JA. Neurosci. Biobehav. Rev. 2012;36:1015. doi: 10.1016/j.neubiorev.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cheeseman JF, Winnebeck EC, Millar CD, Kirkland LS, Sleigh J, Goodwin M, Pawley MD, Bloch G, Lehmann K, Menzel R, Warman GR. Proc. Natl. Acad. Sci. U.S.A. 2012;109:7061. doi: 10.1073/pnas.1201734109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Farrow SN, Solari R, Willson TM. Expert Opin. Drug Disc. 2012;7:535. doi: 10.1517/17460441.2012.689283. [DOI] [PubMed] [Google Scholar]